You’ve probably seen some wild chemistry experiments online, but what you might not realize is that scientists have been capturing the most extraordinary chemical reactions on film for decades. These aren’t just simple color changes or basic bubbling reactions. We’re talking about genuinely bizarre phenomena that seem to defy everything you thought you knew about chemistry.
Some of these reactions look like magic tricks, others resemble living organisms, and a few actually appear to bring matter back from the dead. Get ready to discover reactions that will completely change how you think about the molecular world around you.
The Barking Dog Reaction That Sounds Like Nature

The barking dog experiment involves the reaction of either nitrous oxide with carbon disulfide. As the combustion wave travels down the tube, it compresses the gas ahead of it, giving rise to the characteristic “barking” noise, as well as a bright blue light. You’ll literally hear what sounds like a dog barking from this chemical reaction.
This blast of blue is one of the few examples of chemical luminescence in the gas phase, and is so bright that the reaction was once used as a flash in low-light photography. Imagine discovering that your chemistry experiment could replace actual camera equipment. The reaction produces such intense light that photographers in the early days actually relied on it for illumination in dark conditions.
Molecules Dancing Under the Microscope

Scientists have succeeded in ‘filming’ inter-molecular chemical reactions using the electron beam of a transmission electron microscope as a stop-frame imaging tool. The microscope captured a series of images showing the atoms moving together and apart as they moved along the nanotube. You can actually watch individual atoms form and break bonds in real time.
In one unusual moment, the atoms were split apart, and one settled in a different carbon nook than its partner before returning and forming a bond again. Think about that for a moment. Scientists captured footage of atoms essentially playing a game of molecular musical chairs. This research shows chemical reactions happening in real time at one hundred-millionth of a centimeter.
The Halloween Reaction That Changes Colors on Command

The Old Nassau demonstration is also known as “the Halloween reaction” for reasons that should be readily apparent. Three clear solutions of sodium bisulfate, mercury chloride, and potassium iodate are combined, with the first two solutions mixed together, then added simultaneously to the potassium iodate. What happens next looks like pure Halloween magic.
After a few seconds, the mixtures turn orange-red as mercury iodide precipitates out of solution, then a few seconds later, the mixture rapidly becomes black in color as a starch-iodine complex is formed. The timing is so precise that chemists call this a “clock” reaction. The mixtures have been diluted with increasing amounts of water, which slows the rate of the reactions.
Elephant Toothpaste Explosions That Defy Logic

Elephant’s toothpaste is a hot foamy substance caused by the quick decomposition of hydrogen peroxide using potassium iodide or yeast and warm water as a catalyst. Because it requires only a small number of ingredients and makes a “volcano of foam”, it is a popular experiment for children. Yet what you see on camera often defies all expectations.
At a large scale, Elephant Toothpaste can be a fickle demo; slight variations to the chemistry can increase or decrease significantly, and changing the temperature of chemicals or mixing them longer can be the difference between filling a pool and overflowing it. The reaction is exothermic; the foam produced is hot (about 40-50 degrees Celsius). Scientists have captured reactions that were supposed to create small amounts of foam but instead overflowed entire swimming pools.
The Belousov-Zhabotinsky Spiral Waves That Look Alive

A Belousov-Zhabotinsky reaction is one of a class of reactions that serve as a classical example of non-equilibrium thermodynamics, resulting in the establishment of a nonlinear chemical oscillator. The famous Belousov Zhabotinsky chemical reaction in a petri dish shows pacemaker nucleation sites emit circular waves, and breaking the wavefront with a wire triggers pairs of spiral defects which emit more closely spaced waves.
The concentration of cerium ions oscillates, causing the solution to change between yellow (Ce4+) and colorless (Ce3+) states. Strikingly similar oscillatory spiral patterns appear elsewhere in nature at very different spatial and temporal scales, such as the growth pattern of soil-dwelling amoeba colony, with molecular interactions on a minutes timescale versus single-celled organisms over days to years.
The Mercury Serpent That Grows Like a Living Snake

The hero of this process is Mercury Thiocyanate, a white solid that can be prepared by a precipitation reaction between mercury nitrate or mercury chloride with potassium thiocyanate. The chemical, when ignited, follows a sort of chain reaction releasing smoke and ash and growing into an olive column that looks like a snake, and the color of the serpent can be modified by adding appropriate chemicals.
This reaction is created by burning white powdered mercuric thiocyanate, with the tendril byproducts made of carbon nitride. The materials are highly toxic and the reactions extremely poisonous. Scientists who’ve filmed this reaction describe watching what appears to be a serpent being born from fire, growing longer and more complex by the second.
Oscillating Reactions That Return From the Dead

An oscillating reaction that apparently stops after 10 hours, but is then resurrected spontaneously several hours later, has been discovered by chemistry students. After about 10 hours, the reaction stops and the mixture turns yellow, staying this colour without further change until five to 20 hours have elapsed, at which point the oscillation starts again.
This is the longest flat-lining of a non-linear oscillating reaction, with zombie reactions previously observed only being resurrected after one to two minutes. Imagine watching a chemical reaction that you think has completely died, only to see it spring back to life hours later. The long interval in the oscillating reaction is an example of an entirely new dynamic phenomenon.
The Iodine Clock That Flashes Like Lightning
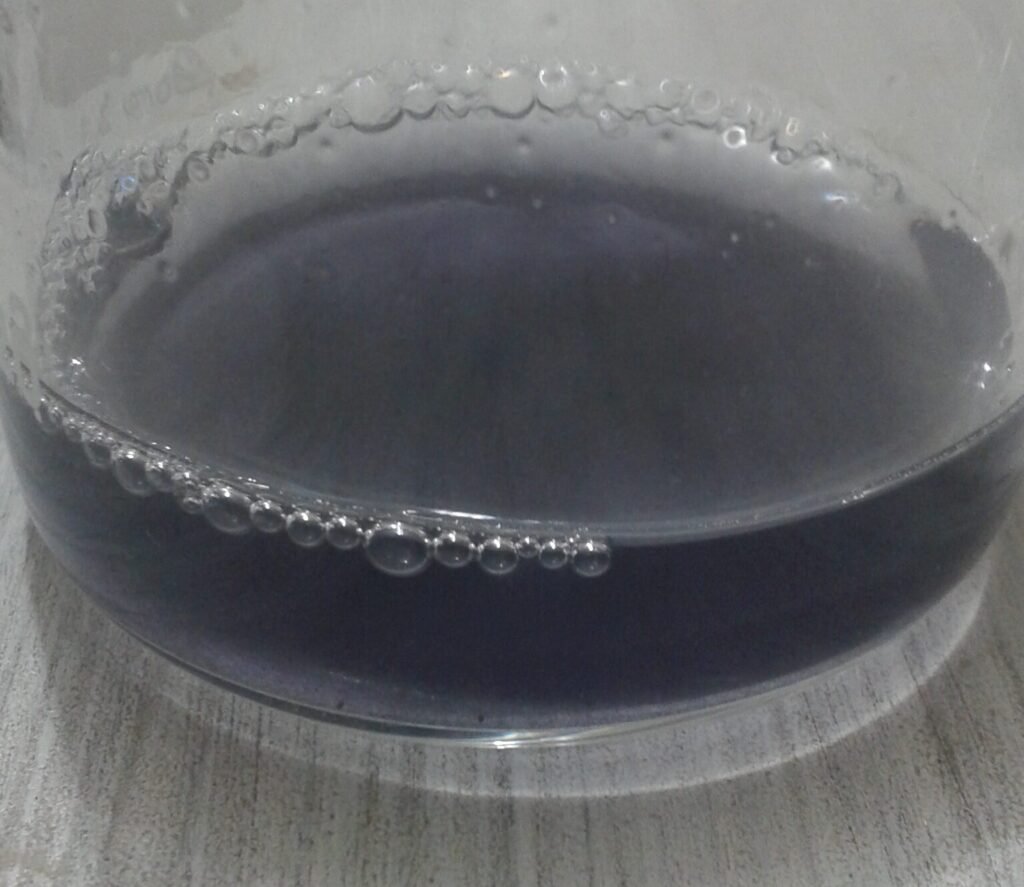
The iodine clock reaction occurs when a solution of hydrogen peroxide is mixed with a combination of potassium iodide, starch and sodium thiosulfate, and after a few seconds the colourless mixture suddenly turns dark blue. The concentration of iodide oscillates between a low and a high value, and each time it increases, some interacts with starch to make a deep blue complex, causing the stirred solution to flash from clear to blue.
There are variations of this experiment where the solution will repeatedly cycle from colourless to blue and back to colourless, until the reagents are depleted. You’re watching a chemical system that can’t seem to make up its mind, switching states with the precision of a metronome until it finally exhausts itself.
The Sugar Snake That Devours Itself

This reaction involves the dehydration of sugar with sulfuric acid, a type of elimination reaction where sugar is a carbohydrate, so when you remove the water from the molecule, you’re left with elemental carbon. The transformation is captured on film as what appears to be a black snake emerging from white sugar, growing larger and more twisted as it consumes the sweet crystals around it.
The visual effect is so striking that many people refuse to believe it’s actually happening. You’re essentially watching sugar turn into carbon right before your eyes, creating a writhing, growing mass that looks more like something from a horror movie than a chemistry lab.
Molecular Shuttles Moving at Nano-Scale

Scientists achieved real-time video imaging of mechanical motions of a single molecular shuttle with sub-millisecond sub-angstrom precision. Using in-situ electron microscopy, researchers produced a video of the assembly of a spherical all-carbon molecule known as a buckyball from a different planar molecule, where it is possible to see the precursor molecule morph into a buckyball as its bonds break and reform.
The changing shape of the molecule from frame to frame gave researchers a good indication of what intermediary forms the molecule took on its way to becoming a buckyball. You’re literally watching a molecule reshape itself into an entirely different structure, like watching origami performed by individual atoms.
Conclusion: Chemistry’s Hidden Cinema

These bizarre chemical reactions captured on camera reveal that the molecular world operates more like a living, breathing theater than the static textbook diagrams we’re used to seeing. From reactions that bark like dogs to molecules that dance together and apart, chemistry proves to be far stranger and more beautiful than most of us ever imagined.
The ability to film these reactions has opened up entirely new ways of understanding how matter behaves at its most fundamental level. Scientists are no longer limited to guessing what happens during chemical transformations. They can watch the molecular drama unfold frame by frame, revealing secrets that were hidden for centuries.
What amazes you most about these captured reactions? Tell us in the comments which one you’d most like to see in person.

Jan loves Wildlife and Animals and is one of the founders of Animals Around The Globe. He holds an MSc in Finance & Economics and is a passionate PADI Open Water Diver. His favorite animals are Mountain Gorillas, Tigers, and Great White Sharks. He lived in South Africa, Germany, the USA, Ireland, Italy, China, and Australia. Before AATG, Jan worked for Google, Axel Springer, BMW and others.