Beneath your feet lies a treasure trove more valuable than any pirate’s chest ever discovered. Deep within Earth’s crust, mantle, and core, elements rarer than gold and more mysterious than ancient artifacts wait in darkness. Some of these hidden treasures power our smartphones, enable medical breakthroughs, and could revolutionize space exploration. Yet most people have never heard their names or understand their incredible potential.
The story of Earth’s rare elements reads like a cosmic detective novel, where stellar explosions billions of years ago scattered precious materials across our planet. While we’ve scratched the surface with mining operations, scientists estimate we’ve only discovered a fraction of the elemental wealth hiding in our planet’s depths. Each rare element tells a unique story of formation, distribution, and the incredible forces that shaped our world.
The Platinum Group Metals Revolution

Hidden deep within Earth’s mantle lie vast deposits of platinum group metals that make today’s known reserves look like pocket change. These elements, including platinum, palladium, rhodium, and iridium, formed during ancient asteroid impacts that delivered cosmic materials to our planet. The largest known deposits exist in South Africa and Russia, but geological surveys suggest enormous untapped reserves remain buried beneath our feet.
Scientists believe the Earth’s core contains more platinum group metals than all surface deposits combined. The challenge lies in accessing these treasures, as they’re locked away thousands of miles below the surface. Recent advances in deep drilling technology and geophysical mapping are revealing new possibilities for discovering these valuable elements closer to the surface.
The demand for platinum group metals continues to skyrocket as industries discover new applications. From catalytic converters that clean our air to cutting-edge fuel cells powering the future, these rare elements are becoming increasingly vital to modern civilization.
Rare Earth Elements: The Invisible Backbone of Technology

Seventeen elements known as rare earth elements form the invisible backbone of virtually every electronic device you touch daily. Despite their name, these elements aren’t actually rare in terms of abundance, but they’re incredibly difficult to extract and process. China currently dominates global production, but vast untapped deposits exist in unexpected places around the world.
Neodymium, dysprosium, and terbium power everything from wind turbines to electric vehicle motors. These elements possess unique magnetic properties that make them irreplaceable in modern technology. The global race to discover new rare earth deposits has intensified as countries recognize their strategic importance for national security and economic stability.
Underground surveys using advanced seismic imaging have revealed promising rare earth deposits in Australia, Canada, and even beneath the ocean floor. The challenge isn’t finding these elements, but developing environmentally responsible extraction methods that don’t devastate local ecosystems.
The Helium-3 Lunar Connection

While most people associate helium with party balloons, a rare isotope called helium-3 could revolutionize energy production and space exploration. This extraordinary element exists in tiny quantities on Earth but appears abundant on the Moon’s surface, deposited over billions of years by solar wind. The potential energy stored in helium-3 could power human civilization for millennia without producing radioactive waste.
Scientists estimate that just 25 tons of helium-3 could power the entire United States for a year. The isotope enables clean nuclear fusion reactions that produce tremendous energy without the dangerous byproducts of traditional nuclear power. This discovery has sparked renewed interest in lunar mining operations and permanent Moon bases.
Recent geological surveys have identified small helium-3 deposits trapped in ancient volcanic rocks and deep underground gas reservoirs. While these terrestrial sources remain extremely limited, they provide valuable research opportunities for understanding this remarkable element’s potential applications.
Lithium: The White Gold Rush

Beneath salt flats, dried lake beds, and ancient brine pools lies lithium, the lightest metal on Earth and the key ingredient in rechargeable batteries. Known as “white gold,” lithium has become one of the most sought-after elements as the world transitions to electric vehicles and renewable energy storage systems. The global lithium market has exploded, with prices reaching unprecedented levels.
The world’s largest lithium reserves exist in a geological formation called the “Lithium Triangle” spanning parts of Argentina, Bolivia, and Chile. These deposits formed over millions of years as ancient seas evaporated, leaving behind concentrated brine pools rich in lithium compounds. Bolivia alone may contain more lithium than the rest of the world combined, but political instability has limited extraction efforts.
New technologies are revolutionizing lithium extraction from unexpected sources. Companies are developing methods to extract lithium from geothermal brines, oil field waste water, and even seawater. These innovations could unlock virtually unlimited lithium supplies and reduce dependence on traditional mining operations.
Cobalt: The Dark Side of the Digital Age

Deep within the Democratic Republic of Congo’s copper mines lies over 60% of the world’s cobalt supply, an element essential for smartphone batteries and electric vehicles. This silvery-blue metal forms under extremely specific geological conditions, making it one of the most geographically concentrated critical elements on Earth. The human cost of cobalt extraction has sparked international concern about ethical sourcing and supply chain transparency.
Cobalt’s unique properties make it irreplaceable in lithium-ion batteries, where it helps prevent overheating and extends battery life. As electric vehicle adoption accelerates, cobalt demand is expected to increase dramatically over the next decade. This surge has prompted mining companies to explore new deposits in Canada, Australia, and the deep ocean floor.
Scientists are racing to develop cobalt-free battery technologies, but current alternatives often sacrifice performance or safety. The search for new cobalt deposits has led to underwater mining proposals that could disturb fragile deep-sea ecosystems. These competing demands highlight the complex relationship between technological progress and environmental protection.
Indium: The Transparent Metal

One of Earth’s rarest elements, indium, makes possible the touchscreen technology that has transformed modern life. This soft, silvery metal is so rare that global annual production measures in hundreds of tons rather than thousands. Indium’s unique ability to conduct electricity while remaining transparent makes it irreplaceable in LCD screens, solar panels, and LED lighting systems.
Most indium comes as a byproduct of zinc refining, making supply dependent on zinc mining operations rather than dedicated indium extraction. China produces about 60% of the world’s indium, creating supply chain vulnerabilities for technology manufacturers. The element’s scarcity has driven prices to extreme volatility, with costs fluctuating dramatically based on supply disruptions.
Recycling initiatives are becoming crucial as indium supplies dwindle. Companies are developing new methods to recover indium from old electronics, but the process remains expensive and complex. Researchers are also exploring indium alternatives, though none match its unique combination of properties essential for modern displays.
Gallium: The Metal That Melts in Your Hand

Gallium presents one of nature’s most fascinating contradictions: a metal that melts at just 85 degrees Fahrenheit, barely above room temperature. This remarkable element hides within aluminum and zinc ores, existing in such small concentrations that it takes tremendous effort to extract meaningful quantities. Despite its scarcity, gallium has become essential for semiconductor manufacturing and advanced electronics.
The global gallium supply depends almost entirely on aluminum production, as gallium extraction requires processing massive amounts of bauxite ore. China dominates both aluminum production and gallium refining, controlling over 80% of the world’s supply. This concentration creates significant supply chain risks for industries dependent on gallium-based semiconductors.
Gallium arsenide semiconductors outperform traditional silicon chips in high-frequency applications, making them essential for 5G communications, satellite technology, and advanced radar systems. As these technologies expand, gallium demand continues to grow despite limited supply sources and recycling challenges.
Tantalum: The Conflict Mineral

Hidden within coltan ore deposits, tantalum has earned the unfortunate nickname “conflict mineral” due to its association with armed conflicts in Central Africa. This extremely corrosion-resistant metal is essential for manufacturing capacitors in electronic devices, from smartphones to medical implants. Tantalum’s unique properties make it nearly impossible to substitute with other materials in critical applications.
The Democratic Republic of Congo contains significant tantalum deposits, but decades of conflict have complicated extraction efforts and raised ethical concerns about mining practices. International certification programs now track tantalum from mine to market, attempting to ensure ethical sourcing. These efforts have improved working conditions but also increased costs for manufacturers.
Australia has emerged as a major tantalum producer, offering conflict-free alternatives to African supplies. New recycling technologies are recovering tantalum from electronic waste, though the process remains expensive and technically challenging. The element’s strategic importance has prompted governments to classify tantalum as a critical material for national security.
Germanium: The Semiconductor Pioneer

Long before silicon dominated the semiconductor industry, germanium powered the first transistors and revolutionized electronics. This metalloid element remains hidden within zinc and copper ores, existing in concentrations so low that it’s often overlooked during mining operations. Despite silicon’s dominance, germanium’s superior electrical properties make it irreplaceable for specialized applications.
Modern fiber optic communications rely heavily on germanium-doped glass, which enables high-speed data transmission across vast distances. The element’s unique optical properties allow precise control of light wavelengths, making global internet communications possible. Space-based solar panels also depend on germanium for maximum efficiency in the harsh environment of space.
Germanium recovery from coal ash and other industrial waste streams has become increasingly important as demand grows. China produces about 60% of the world’s germanium, primarily as a byproduct of zinc refining. The element’s strategic importance for telecommunications and renewable energy has prompted research into new extraction methods and recycling technologies.
Rhenium: The Last Element Discovered

Rhenium holds the distinction of being the last naturally occurring element discovered, found in 1925 by German chemists analyzing molybdenum ores. This extremely rare metal possesses the highest melting point of any element except tungsten, making it invaluable for high-temperature applications. Global rhenium production measures in single-digit tons per year, making it one of the rarest elements in commercial use.
The aerospace industry consumes most of the world’s rhenium supply, using it in superalloys for jet engine components that must withstand extreme temperatures and stress. A single commercial airliner engine may contain several pounds of rhenium, representing thousands of dollars in material costs. The element’s scarcity has driven development of recycling programs to recover rhenium from retired aircraft engines.
Chile produces about 60% of the world’s rhenium as a byproduct of copper mining, with the United States and Canada providing most of the remainder. The element’s extreme rarity and critical applications have made it a strategic material for national defense, prompting governments to maintain strategic reserves despite high costs.
Beryllium: The Lightweight Champion
Beryllium stands out as the lightest structural metal, weighing less than aluminum while maintaining strength comparable to steel. This remarkable element forms under unique geological conditions, primarily in pegmatite deposits where it concentrates over millions of years. The United States produces about 85% of the world’s beryllium, mostly from a single mine in Utah.
Despite its valuable properties, beryllium’s extreme toxicity has limited its applications and made extraction extremely hazardous. Workers must use elaborate protective equipment when handling beryllium, and even small amounts of beryllium dust can cause fatal lung disease. These safety concerns have driven development of alternative materials and recycling programs.
Beryllium’s unique combination of lightness, strength, and thermal conductivity makes it essential for aerospace applications, nuclear reactors, and precision instruments. The element’s scarcity and health risks have made it one of the most expensive metals in commercial use, with prices reaching tens of thousands of dollars per ton.
Tellurium: The Forgotten Semiconductor

Tellurium represents one of the rarest stable elements on Earth, existing in lower concentrations than gold in the Earth’s crust. This metalloid element typically forms as a byproduct of copper refining, where it’s often overlooked or discarded as waste. Despite its scarcity, tellurium has become essential for advanced solar panel technology and next-generation memory devices.
Cadmium telluride solar panels offer some of the highest efficiency rates in commercial photovoltaic technology, making tellurium crucial for renewable energy expansion. The element’s unique properties also enable phase-change memory devices that could revolutionize computer storage systems. These applications have transformed tellurium from an industrial curiosity into a strategically important material.
China dominates global tellurium production, controlling over 50% of the world’s supply through its massive copper refining operations. The element’s extreme scarcity has prompted research into recycling technologies and alternative materials, though none match tellurium’s unique combination of properties essential for advanced applications.
Hafnium: The Nuclear Guardian
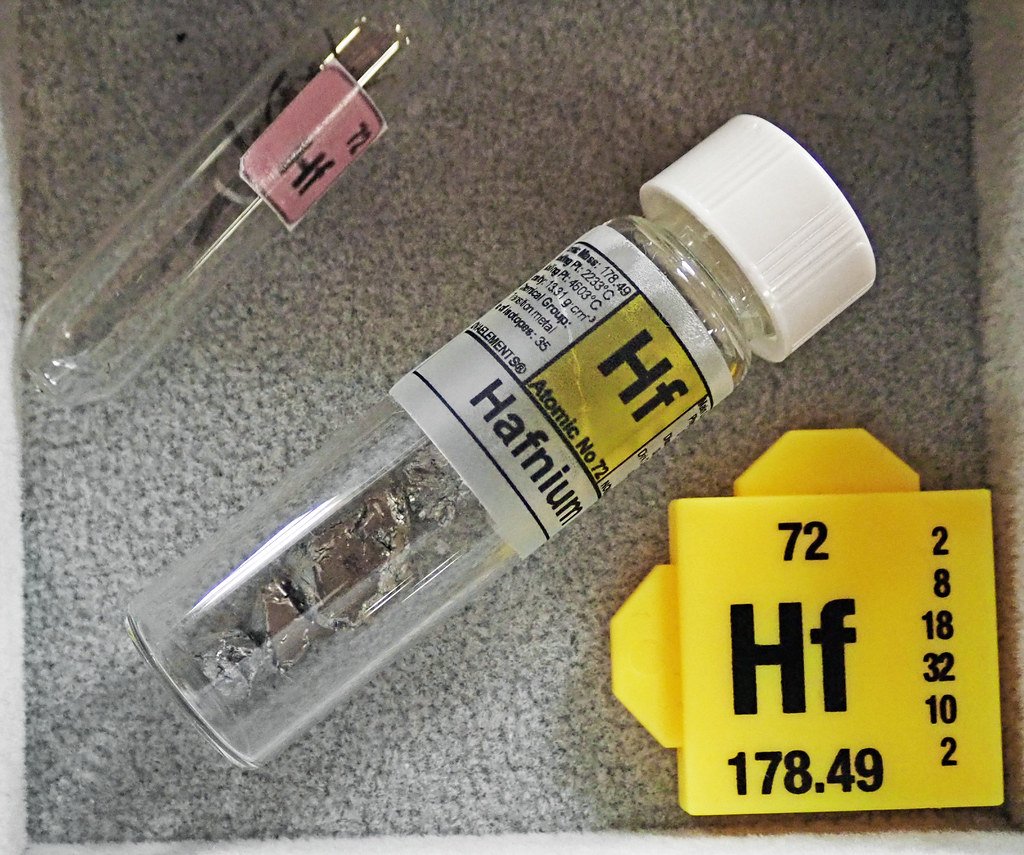
Hafnium serves as one of the most effective neutron absorbers known to science, making it invaluable for nuclear reactor control systems. This dense, silvery metal exists in tiny quantities within zirconium ores, requiring complex separation processes to extract pure hafnium. The element’s nuclear properties make it essential for both civilian power generation and military applications.
Nuclear submarines and aircraft carriers depend on hafnium control rods to regulate nuclear reactions safely and efficiently. The element’s ability to absorb neutrons without becoming radioactive makes it irreplaceable in nuclear applications. Space nuclear reactors also rely on hafnium for radiation shielding and reactor control systems.
Global hafnium production remains limited by the small quantities found in zirconium ores and the expensive extraction processes required. France and the United States produce most of the world’s hafnium, with China emerging as a significant supplier. The element’s strategic importance for nuclear technology has made it subject to export controls and international monitoring.
Osmium: The Density Champion

Osmium claims the title of the densest naturally occurring element, with a density nearly twice that of lead. This platinum group metal forms under extreme conditions deep within the Earth’s mantle, making it one of the rarest elements in the crust. Despite its remarkable properties, osmium’s extreme toxicity and scarcity have limited its commercial applications.
The element’s incredible density and hardness make it valuable for specialized applications requiring extreme durability. Osmium alloys are used in fountain pen nibs, electrical contacts, and precision instruments where wear resistance is crucial. The element’s rarity means global annual production measures in kilograms rather than tons.
Most osmium comes from the same deposits that produce other platinum group metals, primarily in South Africa and Russia. The element’s toxicity requires extreme care during handling and processing, limiting the number of facilities capable of working with osmium. These factors combine to make osmium one of the most expensive elements in commercial use.
Scandium: The Aerospace Alchemist

Scandium transforms ordinary aluminum into aerospace-grade superalloys, creating materials that combine exceptional strength with remarkable lightness. This rare earth element exists in tiny quantities scattered throughout the Earth’s crust, making extraction economically challenging despite its valuable properties. The global scandium market remains small but growing as new applications emerge.
Aerospace manufacturers have discovered that adding small amounts of scandium to aluminum alloys dramatically improves strength, corrosion resistance, and high-temperature performance. These scandium-aluminum alloys could revolutionize aircraft construction, enabling lighter, more fuel-efficient designs. The element’s potential applications extend to sports equipment, where scandium alloys create stronger, lighter baseball bats and bicycle frames.
Australia and China lead global scandium production, though several countries are developing new extraction methods from mining waste and industrial byproducts. The element’s high value and growing demand have prompted research into recycling technologies and alternative supply sources. Some experts predict scandium could become crucial for next-generation transportation systems.
Thulium: The X-Ray Generator

Thulium holds the distinction of being the least abundant rare earth element, existing in concentrations so low that global annual production measures in tens of tons. This silvery metal possesses unique radioactive properties that make it invaluable for portable X-ray devices and medical imaging equipment. The element’s scarcity has made it one of the most expensive rare earth elements in commercial use.
Medical applications consume most of the world’s thulium supply, particularly in portable X-ray machines used for emergency medical care and remote locations. The element’s radioactive isotopes provide precise, controllable radiation sources for medical imaging and cancer treatment. These applications have made thulium essential for modern healthcare despite its extreme rarity.
China produces most of the world’s thulium as a byproduct of rare earth mining and processing operations. The element’s high value and limited supply have prompted research into recycling programs and alternative radiation sources. Some scientists predict thulium could become crucial for next-generation medical imaging technologies.
Lutetium: The Final Frontier

Lutetium represents the final member of the lanthanide series and the rarest of all rare earth elements. This silvery metal exists in such small quantities that global annual production measures in hundreds of kilograms, making it one of the most expensive elements on Earth. Despite its scarcity, lutetium has found specialized applications in medical imaging and research.
Positron emission tomography (PET) scanners rely on lutetium-based detectors for the highest resolution medical imaging available. The element’s unique properties enable precise detection of gamma rays, creating detailed images of internal organs and tissues. These medical applications have made lutetium essential for cancer diagnosis and treatment monitoring.
The extreme rarity and high cost of lutetium have limited research into potential new applications. Most lutetium comes from processing other rare earth elements, where it appears as a trace byproduct. Scientists continue investigating whether lutetium’s unique properties could enable breakthrough technologies in electronics, catalysis, or energy storage.
The Hidden Treasures Beneath Our Feet

Earth’s rare elements represent both incredible opportunities and significant challenges for human civilization. These hidden treasures power our technology, enable medical breakthroughs, and could unlock sustainable energy solutions for future generations. Yet their scarcity, complex extraction processes, and geopolitical concentration create vulnerabilities that could impact global stability.
The race to discover new deposits of rare elements continues as demand grows and traditional sources become depleted. Advanced exploration techniques, from satellite imaging to deep-sea robotics, are revealing previously unknown deposits in unexpected locations. These discoveries could reshape global economics and reduce dependence on traditional mining regions.
Understanding Earth’s rare elements also provides insights into planetary formation and the cosmic processes that created our world. Each element tells a story of stellar nucleosynthesis, asteroid impacts, and geological evolution spanning billions of years. The quest to unlock these hidden treasures continues to drive scientific innovation and human exploration.
As we stand on the threshold of a new technological age, the rare elements hiding beneath our feet may hold the keys to humanity’s future. From clean energy to space exploration, these precious materials could enable breakthroughs that transform civilization. The question isn’t whether we’ll find these hidden treasures, but whether we’ll extract them responsibly and use them wisely. What secrets do you think Earth is still keeping from us?




