Beneath the haunting blue shadows of Antarctic ice, a fish glides through waters colder than your freezer at home. Its heart beats steadily, its fins wave gracefully, and its blood—shockingly—does not freeze. In a world where most life would perish in a heartbeat, these polar creatures have cracked the code of survival, rewriting biology’s rulebook in ways that seem more like science fiction than fact. Imagine walking barefoot across a glacier and feeling nothing but a pleasant chill—this is the kind of impossible feat these animals pull off every day. The secret? A natural antifreeze pulsing through their veins, protecting them from a frozen fate. The more you learn about these wild survivors, the more you realize: nature’s imagination is far wilder than ours.
Life at the Edge: The Brutal Reality of Polar Oceans
Polar oceans are not just cold—they are relentless. Winter darkness can last for months, with temperatures plunging well below zero degrees Celsius. Icebergs drift like floating mountains, and the water is so clear and pure that it almost looks empty. Yet, beneath the surface, life fights a daily battle against freezing. Most fish would stiffen up and die in minutes, their cells torn apart by ice crystals. The polar seas are nature’s ultimate test, demanding powerful strategies just to survive another sunrise. It’s a place where only the most extraordinary adaptations can win. The creatures living here don’t just survive—they thrive where everything else fails. Their existence is a tale of constant risk, resilience, and remarkable ingenuity.
Supercool Survivors: What Makes Antifreeze Fish Unique?
Antifreeze fish aren’t just another quirky oddity of evolution; they are living marvels. Their bodies produce special proteins that keep their blood from solidifying, even when the water around them is colder than ice. This is not a small tweak—it’s a fundamental overhaul of how life works. Imagine your car running perfectly in the Arctic without ever needing to warm up. These fish achieve that on a molecular level, sporting blood that defies the most basic laws of physics. The adaptations don’t stop at their blood, either. Their organs, skin, and even their behavior have shifted to match this icy environment. It’s almost as if they’ve been designed specifically for a world where the rules are completely upside-down.
The Discovery of Antifreeze Proteins: Science Meets Serendipity

The story of how scientists first stumbled upon antifreeze proteins in fish is as surprising as the proteins themselves. In the 1960s, researchers studying Antarctic cod noticed that these fish remained lively in waters so cold they should have been frozen solid. When they looked closer, they realized something incredible was happening inside the fish’s veins. These fish had molecules in their blood that acted like a biological antifreeze, preventing ice from forming. It was a discovery that turned marine biology on its head. Suddenly, researchers were asking new questions: could these proteins help humans preserve organs or make frost-resistant crops? It all started because someone noticed a fish that simply refused to freeze.
How Antifreeze Proteins Work: Nature’s Tiny Shields
Antifreeze proteins are like microscopic superheroes. Instead of letting ice crystals grow and damage cells, these proteins cling to the tiny crystals and stop them from getting bigger. Picture a snowball fight, but every time a snowflake tries to join the ball, a vigilant guard blocks it. These proteins change the physical properties of water, lowering its freezing point and keeping the fish alive even when the temperature drops below zero. Scientists have marveled at how efficiently these proteins work. They only need a tiny amount to make a huge difference. It’s a subtle but powerful defense, a molecular shield that’s both elegant and effective.
Meet the Champions: Notothenioids and Their Frozen Realm

The undisputed stars of the polar fish world are the notothenioids. Found almost exclusively in Antarctic waters, these fish have diversified into dozens of species, each with its own twist on the antifreeze trick. Some are bottom-dwellers, hugging the seafloor in search of food; others dart through open water, quick and agile. What unites them is their shared secret: antifreeze proteins that let them swim freely beneath the ice. These fish have become the backbone of the Antarctic ecosystem, feeding bigger predators and keeping the whole food web in balance. If you want to know what survival looks like in the coldest places on Earth, start with the notothenioids.
Unlocking the Blueprint: Evolution’s Ice-Cold Innovation

The evolution of antifreeze proteins is a story of desperation and genius. Millions of years ago, as the Antarctic cooled and glaciers spread, fish faced a deadly choice: adapt or vanish. Through random mutations, some fish developed proteins that could bind to ice. These lucky survivors passed their genes on, and over time, the antifreeze protein blueprint spread through the population. It’s a perfect example of evolution’s raw creativity, turning a threat into a powerful tool. Today, the genes for antifreeze proteins are so well-tuned that even slight changes can mean life or death. It’s evolution at its most dramatic—a race against the cold, won by a molecular miracle.
Beyond Fish: Other Creatures With Nature’s Antifreeze
Fish aren’t the only ones using antifreeze to beat the chill. Some insects, like the Antarctic midge, have their own version of natural antifreeze. Even certain plants and frogs have developed chemicals to stop their cells from freezing. In Alaska, the wood frog can survive being frozen solid, only to thaw out and hop away in spring. These adaptations show up in the unlikeliest places, proving that nature has a whole arsenal of tricks for life on ice. Each species’ solution is unique, but the goal is the same: avoid turning into a popsicle. It’s a reminder that the struggle against freezing temperatures is a universal challenge—one that sparks endless creativity.
Antarctic Icefish: Blood Without Hemoglobin

If you think antifreeze blood sounds strange, wait until you meet the Antarctic icefish. These ghostly creatures have taken adaptation to a new level—they don’t have hemoglobin, the molecule that gives blood its red color and carries oxygen. Their blood is almost clear, and yet they manage to survive in oxygen-rich polar waters. Scientists believe the cold water holds enough dissolved oxygen to keep them alive, even without the usual red blood cells. This bizarre twist means they can live where few others can, gliding like specters through the icy depths. The icefish are living proof that nature’s solutions often look nothing like what we expect.
Life in Slow Motion: Metabolism and the Cold

In the freezing polar seas, everything slows down—including metabolism. Fish and other creatures here have adapted to burn energy at a snail’s pace, conserving every bit of heat and fuel. This slow-motion lifestyle means they grow more slowly and live longer than their temperate cousins. Their hearts beat fewer times per minute, and their movements are measured, almost careful. It’s a bit like living in perpetual slow-mo, where every action is deliberate. This adaptation isn’t just about survival; it shapes the entire ecosystem, from how fish hunt to how predators stalk their prey. In the polar world, patience is a virtue—and a lifesaver.
The Role of Antifreeze in the Food Web
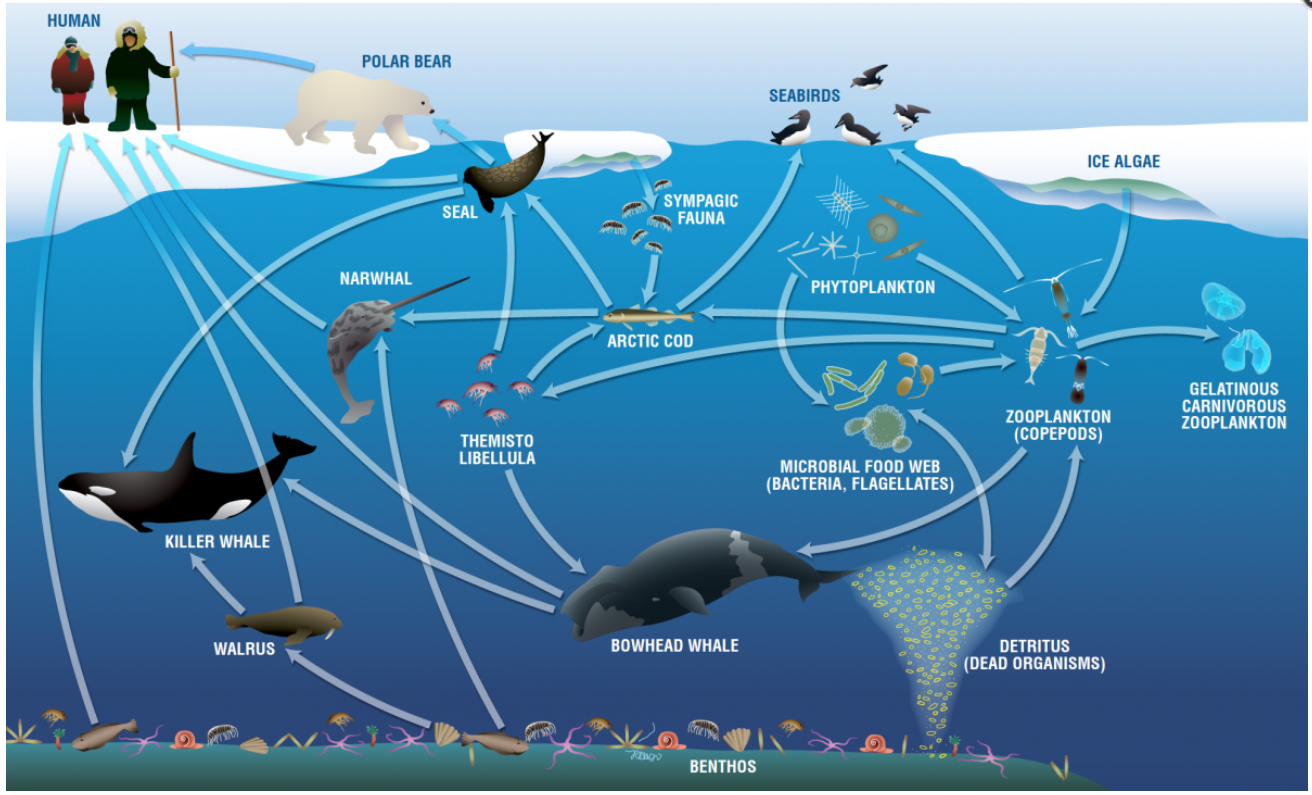
The presence of antifreeze fish doesn’t just help them survive; it supports whole communities of life. By thriving in the coldest waters, these fish provide prey for seals, penguins, and toothed whales. The energy they store and pass along is the foundation for nearly every other animal in the polar seas. Without antifreeze proteins, this vast and intricate web would unravel. It’s a delicate balance, where the survival of one group can mean the difference between abundance and scarcity for countless others. The food web here is a fascinating chain reaction, all kickstarted by the ability to keep blood flowing in a frozen world.
Climate Change: A New Challenge for Antifreeze Fish

Climate change is rapidly reshaping the polar environment. As temperatures rise and sea ice melts, the delicate systems that antifreeze fish depend on are under threat. Warmer waters could mean more competition from outside species, new diseases, or changes in food availability. Some scientists worry that these unique fish, so perfectly adapted to extreme cold, might struggle to cope with a warming world. It’s a dramatic twist in their survival story, forcing them to face challenges they’ve never seen before. The coming decades could reveal whether these icy survivors can adapt once again, or if their miraculous antifreeze will become a relic of the past.
Lessons for Medicine: Antifreeze Proteins in Organ Preservation
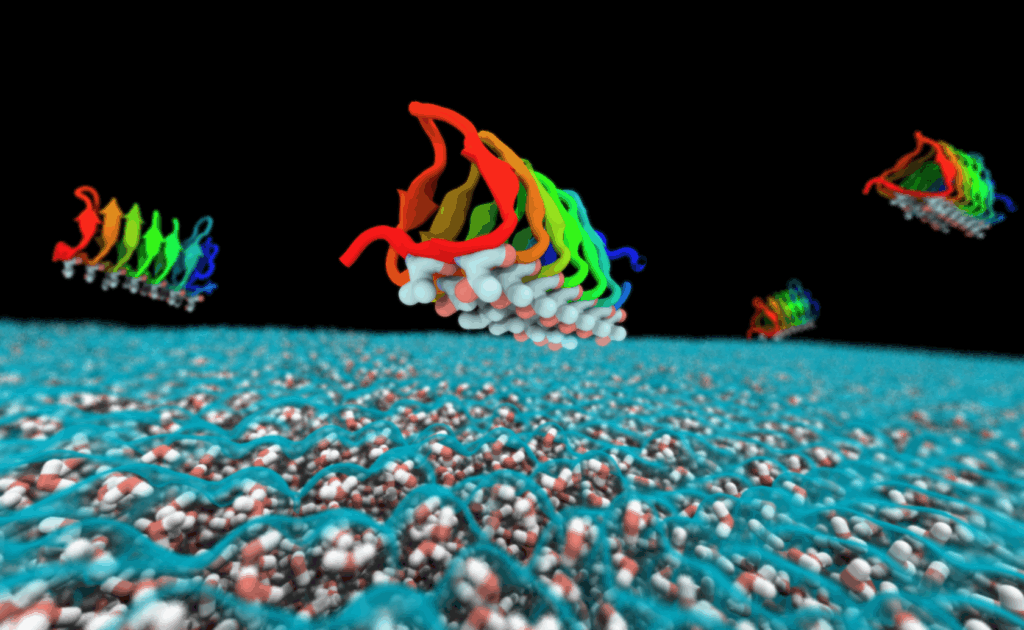
The science behind antifreeze proteins is inspiring new advances in medicine. Doctors and researchers are exploring how these proteins could help preserve human organs for transplant, keeping cells alive at lower temperatures without damage. Imagine being able to store a heart or kidney for days instead of hours, opening up new possibilities for saving lives. The idea comes straight from the Antarctic fish playbook—using nature’s own solutions to solve our biggest problems. It’s a powerful example of how studying wild creatures can lead to medical breakthroughs that help people everywhere.
Engineering Frost-Resistant Crops: Lessons From the Ice
Farmers and scientists are also looking at antifreeze proteins to protect crops from frost. By borrowing genes from polar fish, researchers hope to create plants that can survive sudden cold snaps, reducing losses and increasing food security. These genetically modified crops could be a game-changer in places where unexpected frosts threaten harvests. It’s another case of nature’s ingenuity providing a blueprint for human innovation. The humble antifreeze protein, perfected by fish over millions of years, might one day help feed the world.
The Chemistry of Cold: How Water Behaves Below Zero
Water has some peculiar properties, especially at low temperatures. Unlike most liquids, it expands as it freezes, forming sharp, destructive crystals that can rupture cells. That’s why frostbite is so dangerous for humans and animals alike. Antifreeze proteins change the game by sticking to the edges of forming ice crystals, preventing them from growing and causing harm. It’s a delicate dance at the molecular level, one that scientists are still working to fully understand. The chemistry of cold is a world of hidden dangers—and brilliant solutions.
Symbiotic Relationships: Teamwork in the Frozen Deep
Survival in the polar seas is often a team effort. Some fish form symbiotic relationships with other creatures, like shrimp or bacteria, to get food or protection. In these harsh environments, cooperation can mean the difference between life and death. For example, some species share shelter in icy crevices, taking turns to hunt or rest. These alliances are a testament to the creativity that thrives in the cold. Even in the most isolated corners of the Earth, life finds ways to work together.
Adaptation Beyond Antifreeze: Camouflage and Behavior

Antifreeze proteins aren’t the only trick up polar fish sleeves. Many have developed a stunning ability to blend into their surroundings, using coloration and patterns that match the icy seafloor. Others adjust their behavior, hunting at certain times or swimming in specific ways to avoid predators. Some even migrate short distances to take advantage of slightly warmer waters during critical moments, like spawning. These behavioral adaptations work hand-in-hand with their chemical defenses, creating a complete survival package. It’s a reminder that thriving in the polar world is about more than one secret—it’s a whole arsenal of strategies.
The Price of Survival: Trade-offs and Vulnerabilities

Every adaptation comes with a cost. For polar fish, the energy spent making antifreeze proteins means less energy for other activities, like growing or reproducing. Their slow metabolisms make healing from injuries take longer, and their specialized bodies can’t handle sudden changes in temperature. These trade-offs keep them finely balanced on the edge of survival. It’s a high-stakes gamble, but for millions of years, it has paid off. The price they pay for antifreeze blood is steep, but in the polar oceans, it’s the only way to stay in the game.
What the Future Holds: New Discoveries Await
Scientists are just scratching the surface of what antifreeze fish and other polar creatures can teach us. Every year brings new findings, from unknown species living under the ice to unexpected uses for antifreeze proteins. As technology improves, we can explore deeper and learn more about these mysterious animals. The possibilities are as vast as the polar seas themselves. What new secrets will we uncover? The world of antifreeze blood is still full of surprises, waiting to be revealed.
The story of fish with antifreeze blood is a testament to nature’s ability to find hope in the harshest places. These creatures remind us that survival is not about brute strength, but about cleverness, resilience, and the willingness to change. In a world that is always shifting, those who adapt are the ones who thrive. Isn’t it astonishing how a simple protein can make the difference between life and death?