Picture this: every breath you take, every tree in your backyard, and every grain of sand beneath your feet is part of an ancient dance that’s been choreographed over billions of years. The carbon cycle is Earth’s most vital recycling system, moving carbon atoms through air, water, rocks, and living things in an endless loop that sustains all life on our planet. Yet most people don’t realize how delicate this balance truly is—or how catastrophically things could unravel if we disrupt just one crucial step.
The Invisible Foundation of All Life

Carbon might seem like just another element on the periodic table, but it’s actually the backbone of every living thing on Earth. Without carbon, there would be no proteins, no DNA, no plants, and certainly no humans. This remarkable element has a unique ability to form strong bonds with other atoms, creating the complex molecules that make life possible.
What makes carbon so special is its versatility—it can bond with four other atoms simultaneously, creating everything from simple carbon dioxide to intricate organic compounds. Think of carbon as the ultimate building block, like LEGO pieces that can connect in countless ways to create anything from a simple house to a massive castle.
The carbon cycle ensures that this precious element never gets lost or wasted. Instead, it moves continuously between the atmosphere, oceans, land, and living organisms in a perfectly orchestrated system that has maintained Earth’s climate and supported life for millions of years.
Step 1: The Atmospheric Gateway

The journey begins high above us in the atmosphere, where carbon dioxide floats invisibly around us every second of every day. This colorless, odorless gas makes up about 0.04% of our atmosphere—a tiny fraction that packs an enormous punch. Every molecule of CO2 in the air is like a ticket waiting to be used in the greatest recycling program ever devised.
Atmospheric carbon dioxide doesn’t just sit there passively. It’s constantly moving, mixing with other gases, and waiting for the right moment to join the next phase of its journey. The concentration of CO2 in our atmosphere naturally fluctuates with seasons, weather patterns, and biological activity across the globe.
What’s fascinating is that this atmospheric carbon reservoir contains about 850 billion tons of carbon—sounds like a lot, but it’s actually the smallest of all carbon reservoirs on Earth. This makes it incredibly sensitive to changes, which is why even small increases in atmospheric CO2 can have such dramatic effects on our climate.
Step 2: Photosynthesis – Nature’s Solar Panels

Here’s where the magic really begins. Plants, algae, and certain bacteria perform what might be the most important chemical reaction on Earth: photosynthesis. These green organisms act like living solar panels, capturing sunlight and using its energy to pull carbon dioxide right out of the air and convert it into sugar.
The process is elegantly simple yet mind-blowingly complex. Chlorophyll, the green pigment in plants, absorbs sunlight and uses that energy to combine CO2 from the air with water from the soil. The result? Glucose (plant food) and oxygen (the stuff we breathe). It’s like nature’s own chemistry lab running 24/7 in every leaf around you.
Every single tree in a forest is essentially a carbon-capturing machine, pulling about 48 pounds of CO2 from the atmosphere each year and storing it in their wood, leaves, and roots. Multiply that by the billions of plants on Earth, and you start to understand the massive scale of this natural carbon removal system.
Step 3: The Living Carbon Factories

Once plants have captured carbon through photosynthesis, they become walking, growing carbon storage units. The carbon atoms that were floating freely in the atmosphere just hours ago are now locked away in tree trunks, grass blades, and flower petals. This process is called carbon fixation, and it’s happening all around us every single day.
Plants don’t just store carbon—they build their entire bodies with it. The cellulose in wood, the starches in potatoes, and the sugars in fruits are all made primarily of carbon atoms linked together in different patterns. A massive oak tree might contain several tons of carbon that was once floating in the air as CO2.
But plants aren’t the only living carbon factories. Phytoplankton in the oceans are microscopic powerhouses that collectively remove more carbon from the atmosphere than all the world’s forests combined. These tiny organisms might be invisible to the naked eye, but they’re absolutely crucial to keeping our planet’s carbon cycle in balance.
Step 4: The Food Web Connection

Now comes the part where things get really interconnected. When animals eat plants, they’re not just getting nutrition—they’re participating in a massive carbon transfer system. The carbon that was recently captured from the atmosphere by a blade of grass might soon become part of a rabbit’s muscle tissue, then later part of a fox that ate the rabbit.
Every bite of food represents carbon moving through the food web. When you eat a salad, you’re consuming carbon atoms that were pulled from the air by lettuce leaves just days or weeks ago. When you eat meat, you’re consuming carbon that traveled from plants to animals and now to you.
This biological carbon transfer is incredibly efficient. Animals use some of the carbon for energy (breathing it back out as CO2), store some in their bodies as fat and muscle, and excrete the rest as waste. It’s a complex network where every organism plays a role in moving carbon around the planet.
Step 5: The Breath of Life Returns

What goes in must come out, and that’s exactly what happens with cellular respiration. Every living thing on Earth—from the smallest bacteria to the largest whale—takes in oxygen and releases carbon dioxide as they convert food into energy. This process is essentially photosynthesis running in reverse.
When you breathe out, you’re releasing carbon atoms that might have been part of your breakfast, your lunch, or even food you ate weeks ago. Your body breaks down the carbon-based molecules in food, extracts the energy, and sends the leftover carbon back into the atmosphere as CO2. It’s like a biological recycling program that never stops.
Animals aren’t the only ones doing this. Plants also perform cellular respiration, especially at night when they can’t photosynthesize. They take in oxygen and release CO2, just like animals do. The difference is that plants typically capture more carbon during the day than they release at night, making them net carbon sinks.
Step 6: The Decomposition Highway

When plants and animals die, they don’t just disappear—they become part of nature’s ultimate recycling program. Decomposers like bacteria, fungi, and insects break down dead organic matter, releasing the stored carbon back into the atmosphere as CO2. This process can take anywhere from days to decades, depending on the conditions.
A fallen tree in a forest is like a slow-burning carbon time bomb. Over several years, decomposers will gradually break down the wood, releasing all the carbon that tree spent decades pulling from the atmosphere. The process is so slow you can’t see it happening, but it’s constantly occurring in every forest, field, and backyard around the world.
Soil is actually one of the largest carbon reservoirs on Earth, containing more carbon than the atmosphere and all living plants combined. This soil carbon comes from millions of years of decomposed plant and animal matter, creating a massive underground carbon bank that’s crucial for plant growth and climate regulation.
Step 7: The Ocean’s Carbon Warehouse
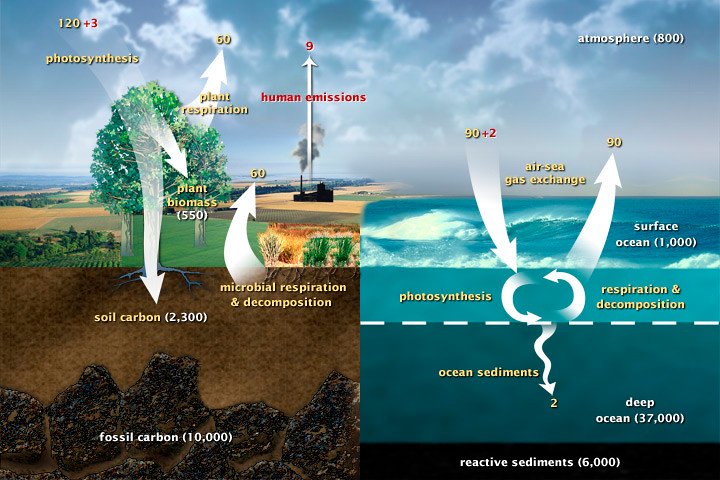
The final step in our carbon cycle journey takes us to the oceans, which serve as Earth’s largest carbon storage system. The ocean absorbs CO2 directly from the atmosphere through a process called oceanic uptake, where gas molecules dissolve into seawater just like bubbles dissolving in a soda.
But the ocean doesn’t just absorb carbon—it actively transports it around the globe through massive underwater currents. Cold water can hold more dissolved CO2 than warm water, so when surface water cools at the poles, it sinks and carries carbon to the deep ocean, where it can remain stored for hundreds or even thousands of years.
Marine organisms also play a crucial role in ocean carbon storage. When tiny sea creatures die, their carbon-rich bodies sink to the ocean floor, where they can be buried and stored for millions of years. This process, called the biological pump, is like a conveyor belt constantly moving carbon from the surface to the deep ocean.
The Hidden Timescales
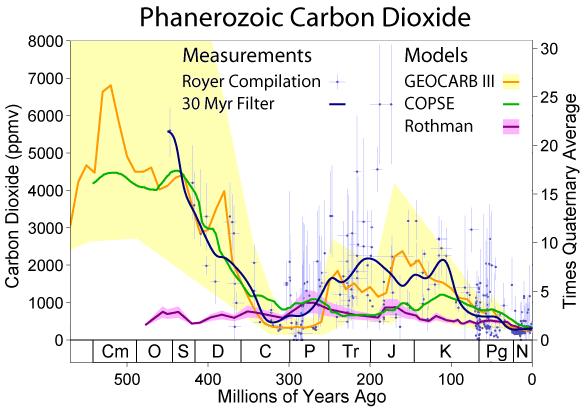
One of the most mind-bending aspects of the carbon cycle is how it operates on completely different timescales simultaneously. Some carbon moves through the cycle in a matter of hours—like the CO2 you breathe out after eating lunch. Other carbon takes geological timescales to complete its journey, remaining locked in rocks and deep ocean sediments for millions of years.
This multi-layered timing system is what keeps Earth’s climate stable over long periods. Fast-cycling carbon responds quickly to changes in biological activity, while slow-cycling carbon provides long-term stability. It’s like having both a sprint and a marathon happening at the same time in the same race.
The carbon in your body right now has likely traveled through countless cycles over Earth’s history. Some of it might have been part of a dinosaur 65 million years ago, spent time as limestone in an ancient sea, and then been released back to the atmosphere through volcanic activity before ending up in the plants you ate for dinner.
When Natural Systems Go Wrong

Even in nature, the carbon cycle isn’t always perfectly balanced. Natural events like volcanic eruptions, massive wildfires, and changes in ocean circulation can temporarily disrupt the normal flow of carbon. These disruptions have happened throughout Earth’s history, sometimes triggering major climate changes and mass extinctions.
The Permian-Triassic extinction event, which occurred about 252 million years ago, was likely caused by massive volcanic eruptions that released enormous amounts of CO2 into the atmosphere. This natural carbon cycle disruption killed about 90% of all species on Earth, showing just how sensitive life is to changes in atmospheric carbon levels.
More recently, the end of the last ice age was triggered partly by changes in how carbon moved between the atmosphere and oceans. As ice sheets melted and ocean currents shifted, the carbon cycle found a new equilibrium that supported the warmer climate we’ve enjoyed for the past 10,000 years.
The Human Factor

For most of human history, we’ve been just another species participating in the carbon cycle. But everything changed when we started burning fossil fuels on a massive scale. Coal, oil, and natural gas are essentially ancient carbon that was removed from the atmosphere millions of years ago and stored underground.
When we burn these fossil fuels, we’re taking carbon that was locked away for geological timescales and releasing it back into the atmosphere all at once. It’s like withdrawing money from a savings account that took millions of years to accumulate and spending it all in a single century.
The numbers are staggering: humans now release about 36 billion tons of CO2 into the atmosphere each year through fossil fuel burning. That’s roughly 100 times more than all the world’s volcanoes combined, and it’s happening at a rate that’s unprecedented in Earth’s history.
The Cascading Effects

What makes the carbon cycle so fragile is that all its components are interconnected. Change one part, and you trigger changes throughout the entire system. As atmospheric CO2 increases, global temperatures rise, which affects plant growth, ocean chemistry, and weather patterns, which in turn affects how carbon moves through the system.
Rising temperatures are causing some carbon sinks to become carbon sources. Arctic permafrost, which has stored carbon for thousands of years, is now thawing and releasing CO2 and methane back into the atmosphere. Warmer oceans are absorbing less CO2, and some forests are releasing more carbon than they’re storing due to increased wildfires and pest outbreaks.
These feedback loops can amplify the original changes, creating a snowball effect that’s difficult to predict or control. It’s like pushing a boulder down a hill—once it starts rolling, it becomes increasingly difficult to stop.
The Ocean’s Changing Chemistry

As the ocean absorbs more CO2 from the atmosphere, it’s undergoing a chemical transformation that could have devastating consequences. When CO2 dissolves in seawater, it forms carbonic acid, making the ocean more acidic. This process, called ocean acidification, is happening at a rate not seen in millions of years.
Ocean acidification affects marine organisms that build shells and skeletons from calcium carbonate, including corals, oysters, and many types of plankton. As the water becomes more acidic, these organisms have trouble building and maintaining their protective structures, which can disrupt entire marine ecosystems.
The implications go far beyond just marine life. If ocean acidification severely impacts phytoplankton—the tiny organisms that form the base of the marine food web and produce much of the world’s oxygen—it could affect the entire planet’s ability to support life. We’re essentially conducting a global chemistry experiment with unpredictable results.
Forest Fires and Carbon Bombs

Climate change is turning some of our most important carbon sinks into carbon bombs. Forests that have stored carbon for decades or centuries can release it all back into the atmosphere in a matter of hours during a wildfire. As temperatures rise and droughts become more common, these fires are becoming larger and more frequent.
The 2020 wildfire season in Australia released an estimated 715 million tons of CO2 into the atmosphere—equivalent to the annual emissions of a large industrialized country. These fires didn’t just burn trees; they also burned soil organic matter that had been accumulating for thousands of years, releasing ancient carbon back into the atmosphere.
What’s particularly concerning is that many of these burned forests may not grow back the same way, especially if the climate has changed too much to support the same types of trees. Instead of being carbon sinks, these areas might become permanent carbon sources, fundamentally altering the global carbon cycle.
The Permafrost Time Bomb

Hidden beneath the frozen soils of the Arctic lies one of the largest carbon reservoirs on Earth—permafrost. This permanently frozen ground contains about twice as much carbon as the entire atmosphere, locked away in organic matter that has been preserved for thousands of years. But as global temperatures rise, this ancient carbon storage system is beginning to thaw.
When permafrost thaws, the organic matter it contains begins to decompose, releasing CO2 and methane into the atmosphere. Methane is particularly concerning because it’s about 25 times more potent than CO2 as a greenhouse gas. It’s like a massive, slow-motion explosion of greenhouse gases that could continue for decades or centuries.
Scientists estimate that permafrost could release between 130 and 160 billion tons of carbon by 2100—roughly equivalent to adding another United States worth of emissions to the atmosphere. This represents a fundamental shift in the carbon cycle, turning a long-term carbon sink into a major carbon source.
Technology’s Role in the Cycle

While human activities have disrupted the carbon cycle, technology might also offer solutions. Carbon capture and storage technologies are being developed to remove CO2 directly from the atmosphere and store it safely underground. These systems essentially mimic what plants do naturally, but at a much faster rate.
Some of the most promising technologies involve enhancing natural carbon sinks. Reforestation and afforestation programs can rapidly increase the amount of carbon stored in vegetation, while regenerative agriculture practices can help soils store more carbon. These approaches work with the natural carbon cycle rather than against it.
Advanced technologies like direct air capture and artificial photosynthesis are still in their early stages, but they could eventually play a major role in rebalancing the carbon cycle. The key is scaling these technologies fast enough to make a meaningful difference before tipping points are reached.
The Tipping Point Reality

Perhaps the most frightening aspect of carbon cycle disruption is the concept of tipping points—thresholds beyond which changes become irreversible or self-reinforcing. These aren’t gradual transitions; they’re sudden shifts that can happen within decades or even years once certain conditions are met.
Scientists have identified several potential tipping points in the carbon cycle, including the collapse of the Amazon rainforest, the shutdown of ocean circulation patterns, and the complete melting of polar ice sheets. Each of these events could trigger massive releases of stored carbon, fundamentally altering Earth’s climate system.
The scary part is that we don’t know exactly where these tipping points are until we cross them. It’s like walking blindfolded toward a cliff—you don’t know how close you are to the edge until you step off. Once triggered, these changes could continue for centuries or millennia, regardless of what humans do.
Signs of System Stress
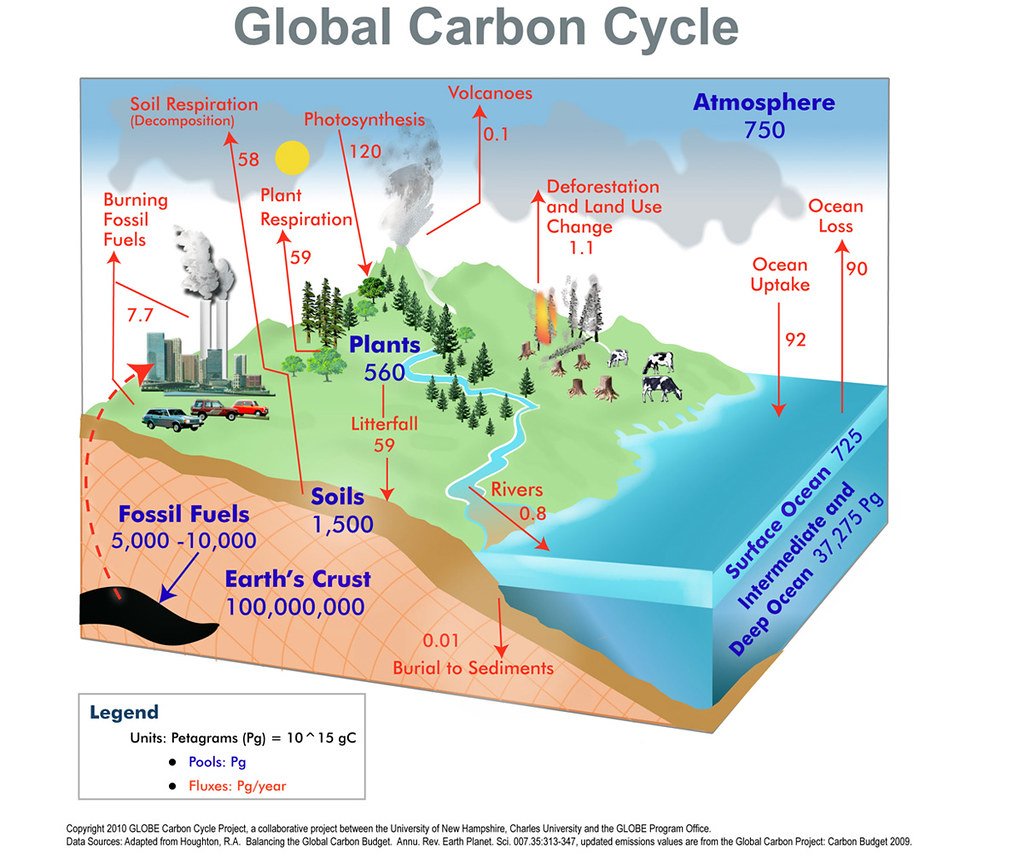
The carbon cycle is already showing signs of stress from human activities. Atmospheric CO2 levels are now higher than they’ve been in over 3 million years, and they’re rising at an unprecedented rate. The annual increase in atmospheric CO2 has accelerated from about 1 part per million per year in the 1960s to over 2 parts per million per year today.
Natural carbon sinks are becoming less effective at absorbing human emissions. The fraction of human CO2 emissions that stay in the atmosphere has increased from about 40% in the 1960s to about 45% today. This means that natural systems are becoming saturated and less able to buffer our emissions.
Seasonal variations in atmospheric CO2 are becoming more extreme, particularly in northern latitudes. This suggests that ecosystems are responding to changing conditions in ways that amplify the natural carbon cycle, potentially making the system more volatile and unpredictable.
The Interconnected Web

Understanding the carbon cycle requires recognizing that it’s not isolated from other Earth systems. The carbon cycle is intimately connected to the water cycle, the nitrogen cycle, and countless other natural processes. Disrupting one affects them all, creating cascading changes that can be difficult to predict or control.
Climate change driven by carbon cycle disruption is already affecting water availability, which in turn affects plant growth and carbon storage. Changes in precipitation patterns can turn carbon sinks into carbon sources, while rising temperatures can accelerate decomposition and release more stored carbon.
The interconnected nature of these systems means that solutions must be holistic rather than piecemeal. We can’t fix the carbon cycle without considering its relationships with other Earth systems, and we can’t address climate change without understanding how all these cycles work together.
What Breaking the Cycle Really Means

When we talk about “breaking” the carbon cycle, we’re not talking about stopping it entirely—that’s impossible. Instead, we’re talking about pushing it so far out of its natural balance that it can no longer support the stable climate conditions that have allowed human civilization to flourish. The cycle will continue, but in a new configuration that might be hostile to life as we know it.
A broken carbon cycle could mean runaway climate change, where increasing temperatures trigger more carbon releases, which cause more warming, in an unstoppable feedback loop. It could mean the collapse of ecosystems that have existed for millions of years, the extinction of countless species, and the transformation of Earth into a planet that’s barely recognizable.
The most terrifying aspect is that once certain thresholds are crossed, the changes become irreversible on human timescales. We might be able to stop adding carbon to the atmosphere, but we can’t quickly remove the carbon that’s already there, and we can’t restart natural systems that have collapsed.
The carbon cycle is far more than just a scientific concept—it’s the life support system that makes our planet habitable. Every step in this seven-part dance is crucial, and disrupting any one of them can trigger cascading changes throughout the entire system. We’re currently conducting the largest uncontrolled experiment in Earth’s history by rapidly altering atmospheric carbon levels, and the results are already becoming visible in changing weather patterns, melting ice, and shifting ecosystems. The window for maintaining the stable climate conditions that have supported human civilization is rapidly closing, but understanding how the carbon cycle works gives us the knowledge we need to act. The question isn’t whether we can fix what we’ve broken, but whether we have the will to do it before it’s too late. What future are we choosing for the next generation?




