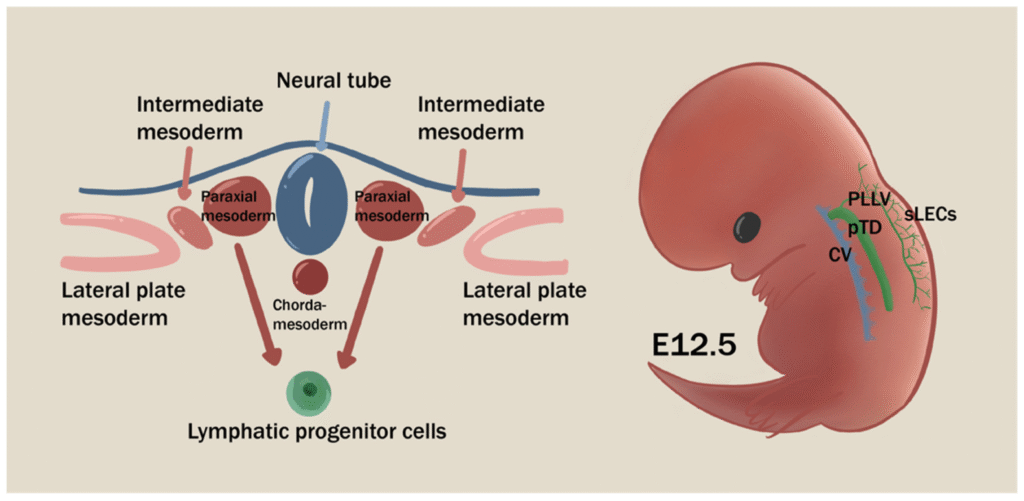
A protective balloon around every mammal begins as a whisper of tissue, invisible to the naked eye and largely off-limits to science. Researchers are working to develop methods for coaxing human stem cells to assemble structures that could mimic crucial weeks after implantation – an elusive window of development that’s long been a black box. Earlier models hinted at this moment, but they faded quickly; these new structures last, organize, and talk to neighboring cells in ways that matter. The result isn’t a baby-in-a-dish, but it is a durable, testable slice of early life that could rewrite how we study pregnancy and its risks. It’s a breakthrough built on decades of patient tinkering – and it changes the questions we’re finally able to ask.
The Hidden Clues
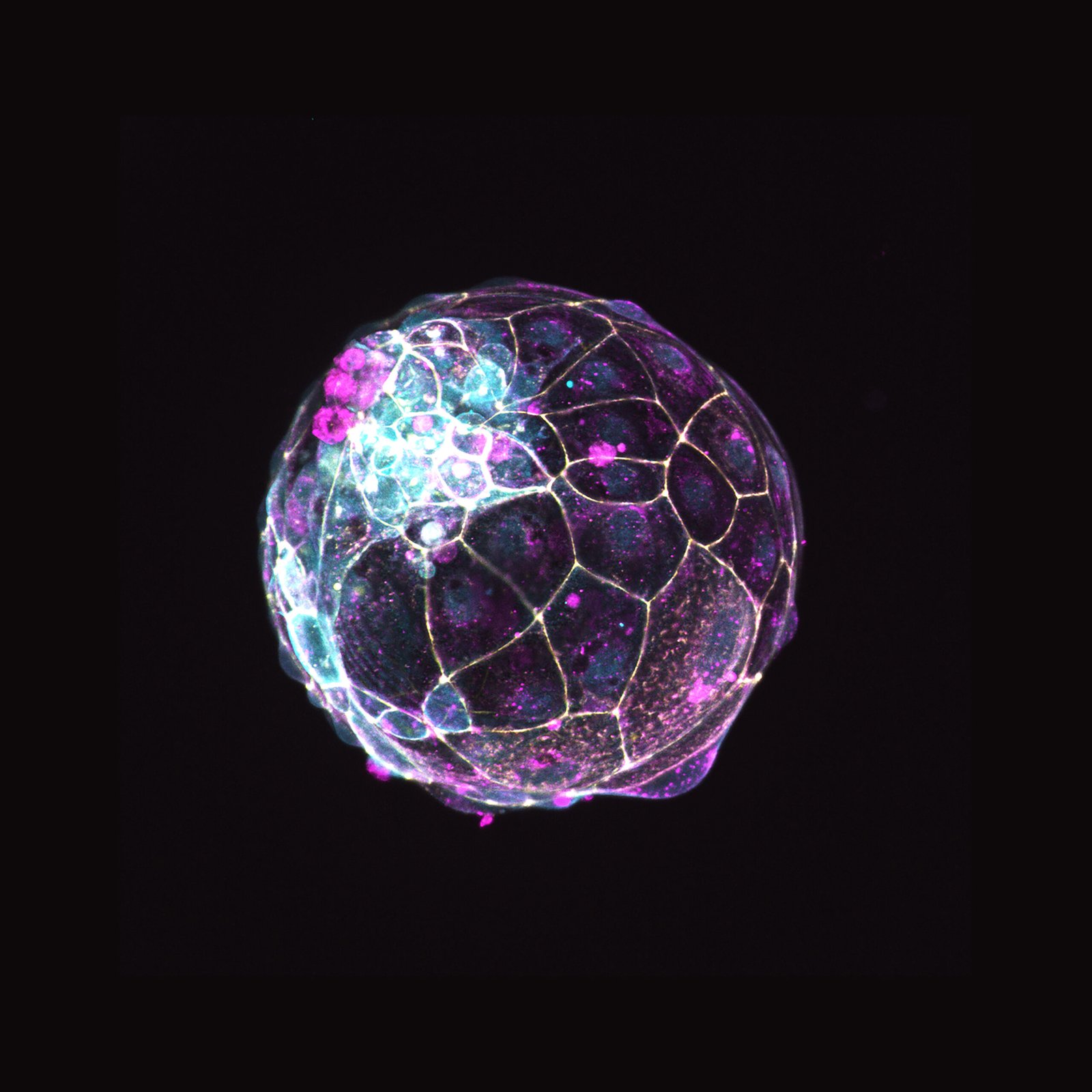
For years, scientists could observe either very early embryos or late pregnancy tissues, but the amnion’s transformation between the second and fourth week stayed largely hidden. Proposed stem cell models could potentially fill that gap by recreating sac-like structures that resembles the mature amnion and its neighboring layers. In theoretical culture conditions, such sacs could potentially form and expand, providing a canvas for experiments that were once impossible. This matters because the amnion isn’t just a passive cushion; it signals, shapes, and supports the embryo during a narrow window of development. Until now, that signaling choreography was mostly guesswork inferred from animal studies and rare human samples. Researchers finally have a system that shows the amnion’s own voice in the conversation of early life.
Just as telling, these models move research beyond the fourteen-day limit that governs embryo studies without breaching that ethical boundary. They are derived from stem cells and build the amnion and associated extra-embryonic tissues, not full embryos. That distinction lets scientists probe late-implantation biology with precision. The field has long needed that clarity – and this platform delivers it with reproducibility, scale, and a roadmap for targeted tests.
How Scientists Did It
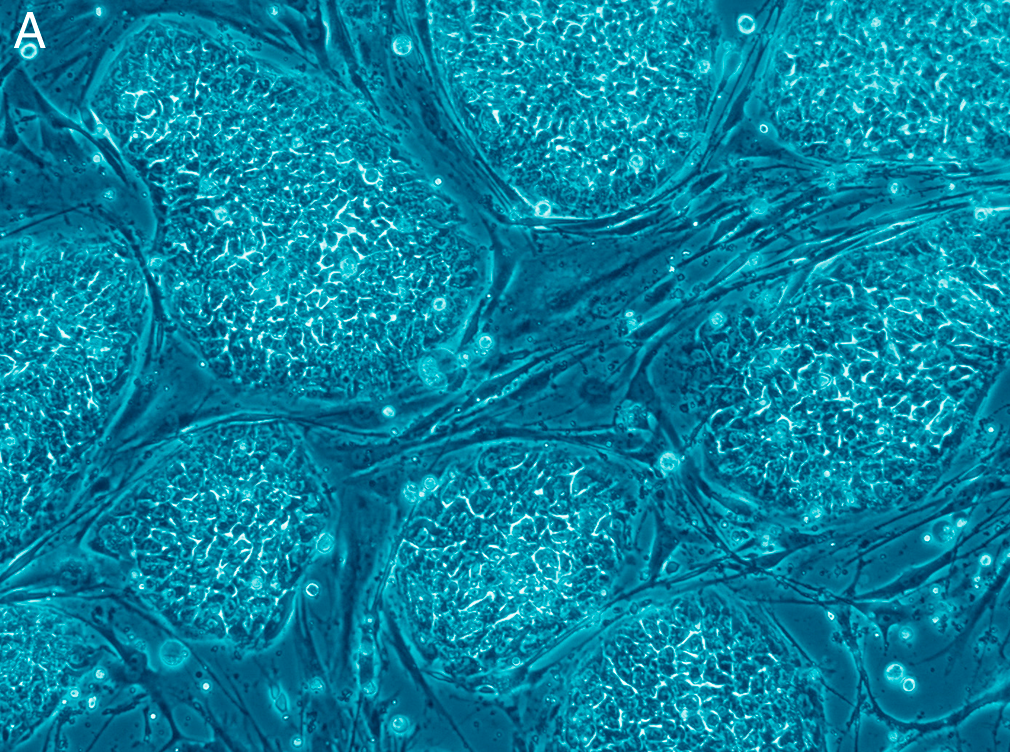
The recipe sounds almost too simple: expose human embryonic stem cells to two well-chosen signals for about two days, then wait as the cells self-organize. In theoretical protocols, a sac could potentially take shape within roughly ten days in cultures, developing distinct layers that match what’s seen in the amniotic membrane inside the uterus. Over the following weeks, the sacs grow and the fluid inside begins to mirror the chemistry of true amniotic fluid. It’s a careful choreography of timing, concentration, and cell density, tuned so the tissue architectures emerge on their own. That self-assembly is the headline, but the backstage star is a transcription factor that flips the developmental switch. Toggle it down, and amnion formation sputters; nudge it up, and the program kicks into gear.
That switch – centered on the gene GATA3 – acts like a conductor for amnion identity. Its role links signaling pathways to structural outcomes, offering a handle to test how the amnion gets built and maintained. Even better, the workflow scales, making it practical to run many conditions in parallel. For developmental biology, that’s the difference between pretty pictures and hard, testable rules.
What the Mini-Sacs Revealed
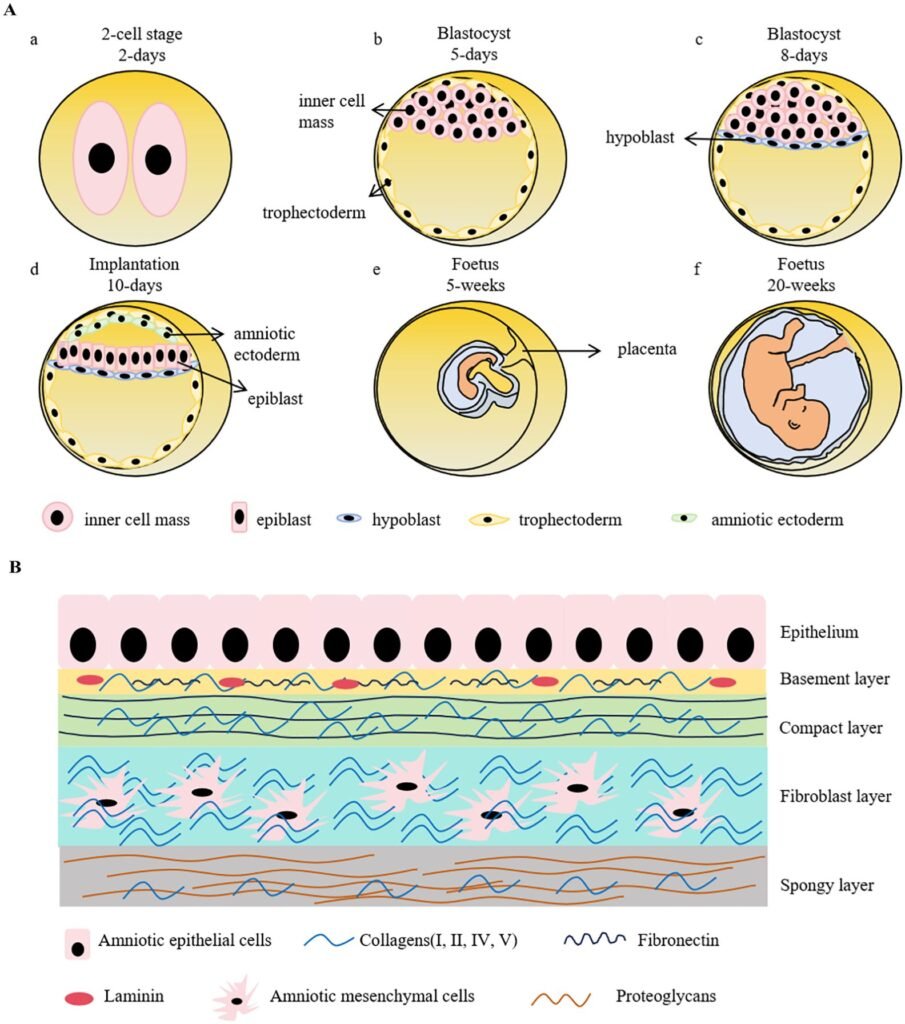
Once the sacs were stable, the team asked whether they could do more than just look the part. They do: the amnion models emit cues that nudge nearby cells toward specific fates, underscoring that this tissue participates in guiding development, not merely shielding it. The sacs also generate supportive tissues seen in early pregnancy, painting a fuller picture of the extra-embryonic environment. Because the setup is tractable, researchers can tweak nutrition, oxygen levels, or signaling molecules and watch how the system responds in days, not months. In practical terms, that means we can start to map which changes derail normal growth and which ones the amnion can buffer. Those insights could explain why some pregnancies falter early, while others adapt and thrive.
As a reporter who once sat through countless talks of fragile “blastoids” and short-lived cysts, I didn’t expect these sacs to last or to talk back to neighboring cells so convincingly. Seeing longevity and function in the same model feels like switching from grainy radio to high-definition audio. It’s the difference between guessing and hearing the notes clearly.
Why It Matters
Traditional approaches relied on scarce donated tissues or brief embryo models that captured only the earliest patterns before day fourteen. By contrast, these lab-made sacs capture the amnion’s mature phase, enabling experiments on how mechanical forces, infections, toxins, or maternal health conditions might alter its behavior. That opens doors for understanding early miscarriage, preeclampsia risk pathways, and disorders tied to amniotic membrane failure. It also offers a controlled platform to compare how common medications or environmental exposures ripple through early pregnancy biology. The vast majority of pregnancy complications trace back to events in the first trimester, yet detailed human data from that period have been thin. This model is a direct route to the mechanisms we’ve only inferred until now.
There’s also a translational angle: surgeons and ophthalmologists already use amniotic membranes for wound healing and corneal repair, but supply and consistency vary. Scalable, standardized amnion-like tissue could reduce dependence on donor sources and improve quality control. If validated, it might speed up trials for bioactive membranes that release precise growth factors. The signal-to-structure link via GATA3 adds a molecular dial to tune performance, a rarity in current biomaterial sourcing.
Lines We Won’t Cross
Ethics isn’t a footnote here; it’s the frame. These amnion models are not embryos, and they do not develop into fetuses, but they simulate a critical, previously inaccessible slice of human development. That lets researchers work beyond the fourteen-day embryo-study limit without violating it, a boundary set to balance scientific value and moral caution. The approach leverages extra-embryonic tissues derived from stem cells to study signaling, structure, and failure modes in a controlled way. After years of debate about embryo-like models, the field is converging on definitions that respect limits while enabling discovery. Clarity about what these sacs are – and are not – is foundational to maintaining public trust.
Regulators and ethics boards will still watch closely, especially as teams push toward patient-specific versions. Transparent reporting and pre-registered protocols can keep the work on steady ground. The prize is real: insight into a stage of life that medicine has struggled to see, let alone fix when it goes wrong.
Global Perspectives
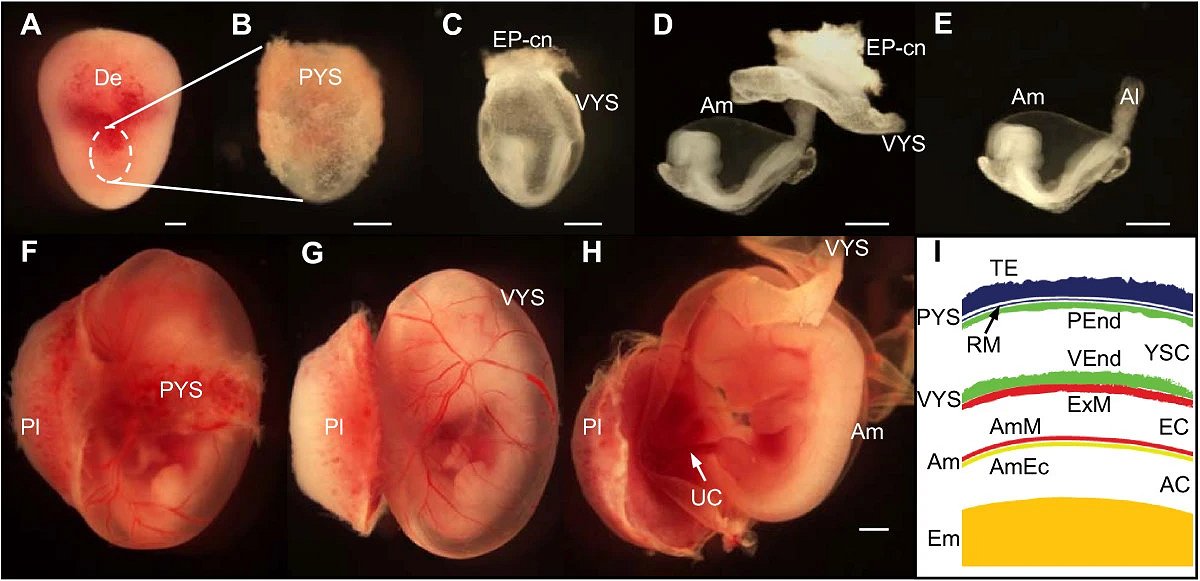
This advance didn’t arrive in a vacuum; it builds on earlier amniotic sac-like systems that formed around implantation but were shorter-lived and less complete. Those predecessors proved human stem cells could self-organize into amniotic ectoderm and epiblast-like regions, hinting at the choreography now captured more fully. By adding durability, layered structure, and signaling readouts, the new models shift from proof-of-concept to usable platform. They complement parallel breakthroughs that grow organoids from amniotic-fluid cells during pregnancy, creating personalized tissues for diagnosis and drug testing. Taken together, the toolbox for studying early life is diversifying rapidly, with models that range from embryo-adjacent structures to patient-specific organoids. The common thread is precision without breaching ethical lines.
Different countries calibrate oversight differently, but the core scientific questions transcend borders: how do signals flow, when do membranes fail, and which interventions actually help? As labs adopt shared standards, data from London to Denver can align into a coherent picture rather than scattered case reports. Coordinated biobanking and open protocols will make the work faster and fairer. That cooperation matters, because early pregnancy loss and complications are both universal and understudied.
The Future Landscape
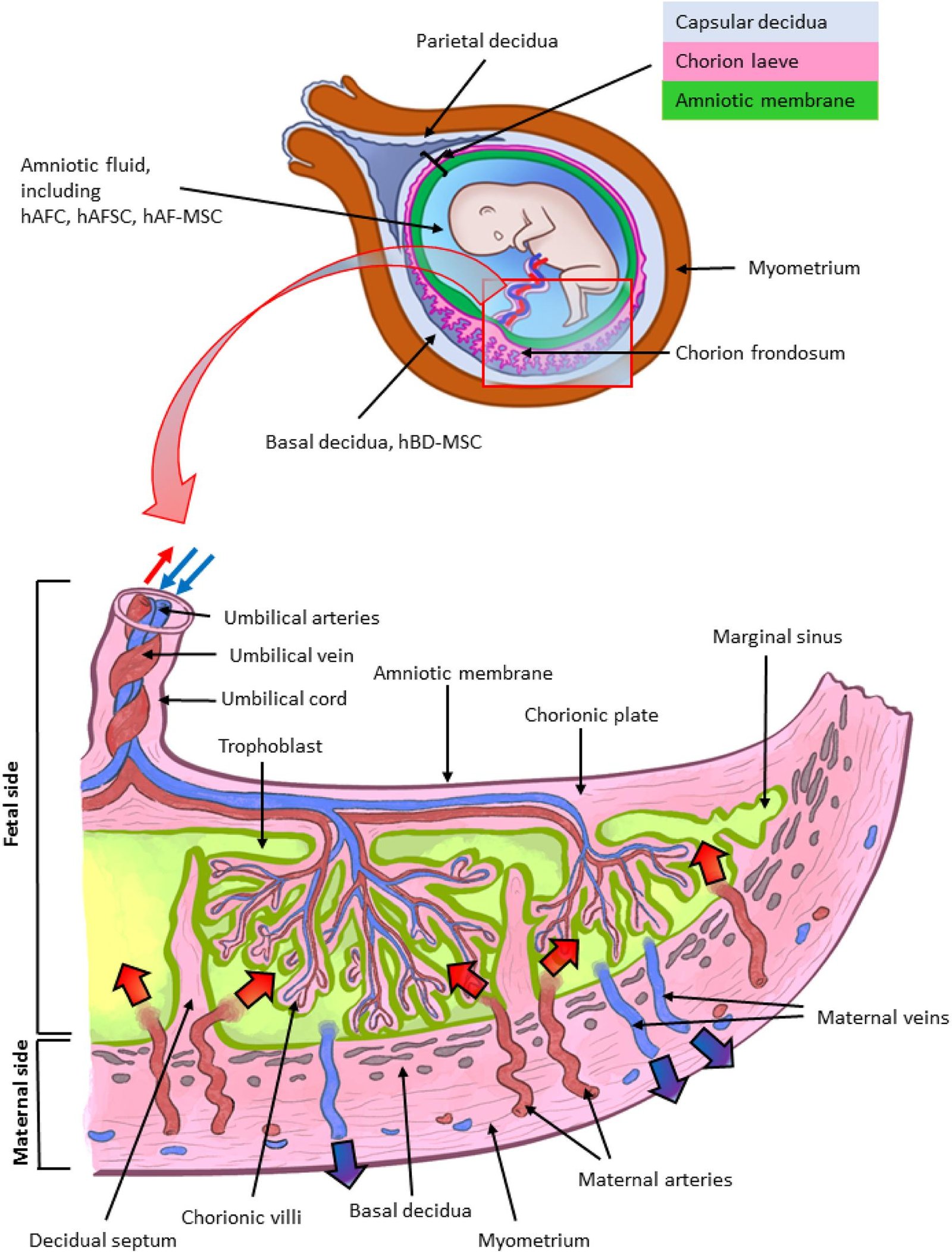
The next frontier is personalization: generating amniotic sacs from induced pluripotent stem cells, so scientists can study patient-specific risks before pregnancy or early in gestation. Pair that with single-cell multi-omics and live biomechanics, and we could map how gene variants, diet, or infections tip the amnion from supportive to stressed. Clinically, safer ways to collect and bank amniotic-fluid cells – even at delivery – could feed rapid diagnostics and reparative therapies. Imagine screening candidate drugs directly on a patient-matched amnion model to flag benefits or risks within days. Just as importantly, scaled production could supply consistent amniotic membranes for reconstructive medicine, relieving bottlenecks in surgery. The work is early, but the path toward translation is clearer than it’s ever been.
Risks remain: over-interpretation, uneven regulation, and the temptation to frame these sacs as more than they are. The responsible move is careful benchmarking against human tissue and open sharing of failures. That’s how this discovery becomes a reliable foundation, not a one-off headline.
Take Part: What You Can Do

If this research resonates, there are practical ways to help it move forward responsibly. Support institutions that prioritize transparent, ethically reviewed stem cell science, and stay informed as guidelines evolve. If you’re pregnant or planning to be, consider participating in registries or studies that monitor environmental exposures and outcomes – data that improve these models’ relevance. Clinicians and educators can fold these findings into discussions about early pregnancy care, especially around medication safety and infection prevention. Funders and policymakers can back open-access datasets and cross-border consortia so results are comparable and reproducible. Small moves from many people add up to a research ecosystem that’s safer, faster, and more equitable.

Suhail Ahmed is a passionate digital professional and nature enthusiast with over 8 years of experience in content strategy, SEO, web development, and digital operations. Alongside his freelance journey, Suhail actively contributes to nature and wildlife platforms like Discover Wildlife, where he channels his curiosity for the planet into engaging, educational storytelling.
With a strong background in managing digital ecosystems — from ecommerce stores and WordPress websites to social media and automation — Suhail merges technical precision with creative insight. His content reflects a rare balance: SEO-friendly yet deeply human, data-informed yet emotionally resonant.
Driven by a love for discovery and storytelling, Suhail believes in using digital platforms to amplify causes that matter — especially those protecting Earth’s biodiversity and inspiring sustainable living. Whether he’s managing online projects or crafting wildlife content, his goal remains the same: to inform, inspire, and leave a positive digital footprint.