For years, organoids – those tiny, lab-grown versions of human organs – have been impressive but incomplete, like movie sets without working plumbing. The weakest link was life’s most basic requirement: flow. Without blood vessels, organoids stalled at sesame-seed size and starved in their cores, limiting what scientists could learn. Recent research has begun to address this logjam as researchers stitched living vasculature into beating cardiac tissues and even coaxed miniature lungs to grow their own vessel networks. The result is a new generation of mini-organs that don’t just twitch; they move fluids, sustain themselves more deeply, and hint at models that one day could push red cells through capillaries on command.
The Hidden Clues
What if a human heart the size of a pea could push fluid through tiny vessels – on a lab bench? That’s the provocative image behind this wave of breakthroughs, and it lands with a jolt because it flips the old organoid story on its head. Instead of fragile clusters that beat at the surface and fade in the middle, researchers are building tissues with internal highways for oxygen, nutrients, and waste. The moment you add plumbing, biology behaves differently: cells mature further, rhythms stabilize, and drug responses look more like the real thing. This shift isn’t just technical; it’s conceptual, reframing organoids from pretty models to working systems. For me, the first time I watched a video of perfused tissue, it felt like seeing a dry riverbed suddenly fill after a storm.
From Petri Dish to Pulse
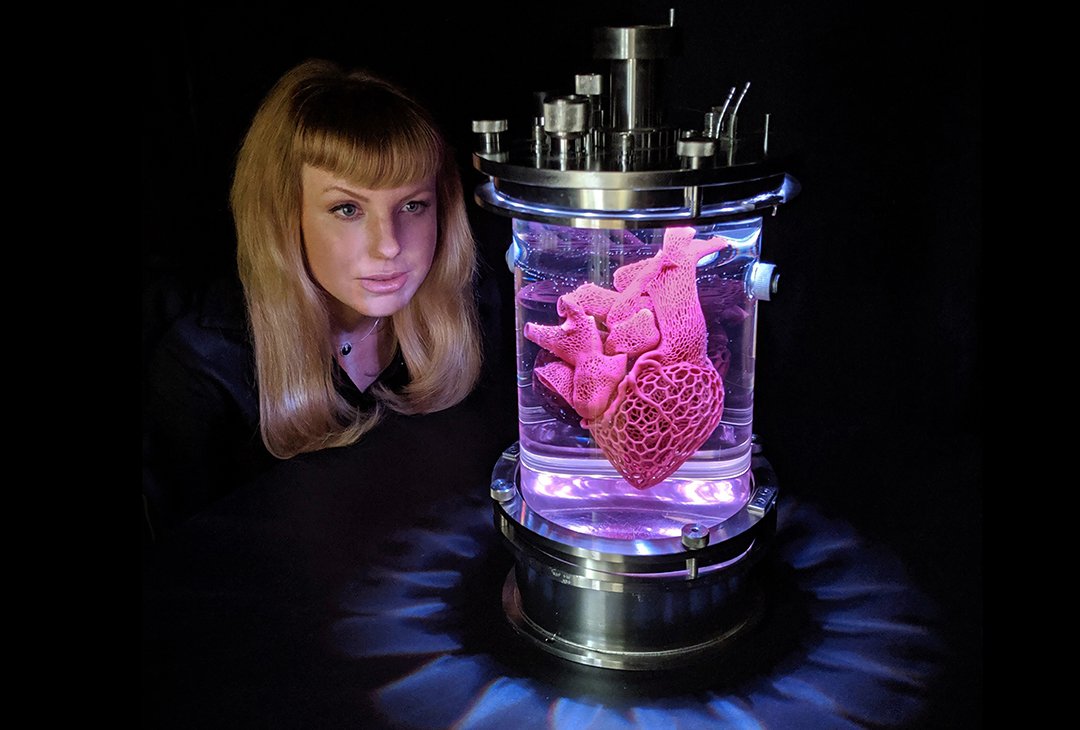
Researchers have been working on heart and liver organoids that developed intricate, organ-appropriate blood vessels, tackling the field’s most stubborn size limit. By carefully timing molecular cues and spatial patterns, they guided stem cells to form cardiomyocytes, supportive smooth muscle, and endothelial cells that assembled into branching networks, while the heart tissue beat rhythmically in culture. The approach didn’t just add tubes; it recapitulated an early-stage choreography of development, allowing tissues to organize with more realism.
These vascularized heart organoids resemble an embryonic-stage blueprint, offering a controlled, human model for early development and potentially safer drug testing. Although the vessels are not yet routinely used to carry full-flow media over long timescales in all labs, the roadmap is there and already being iterated. That alone marks a decisive step past the old plateau of static, starved organoids.
Flow, For Real
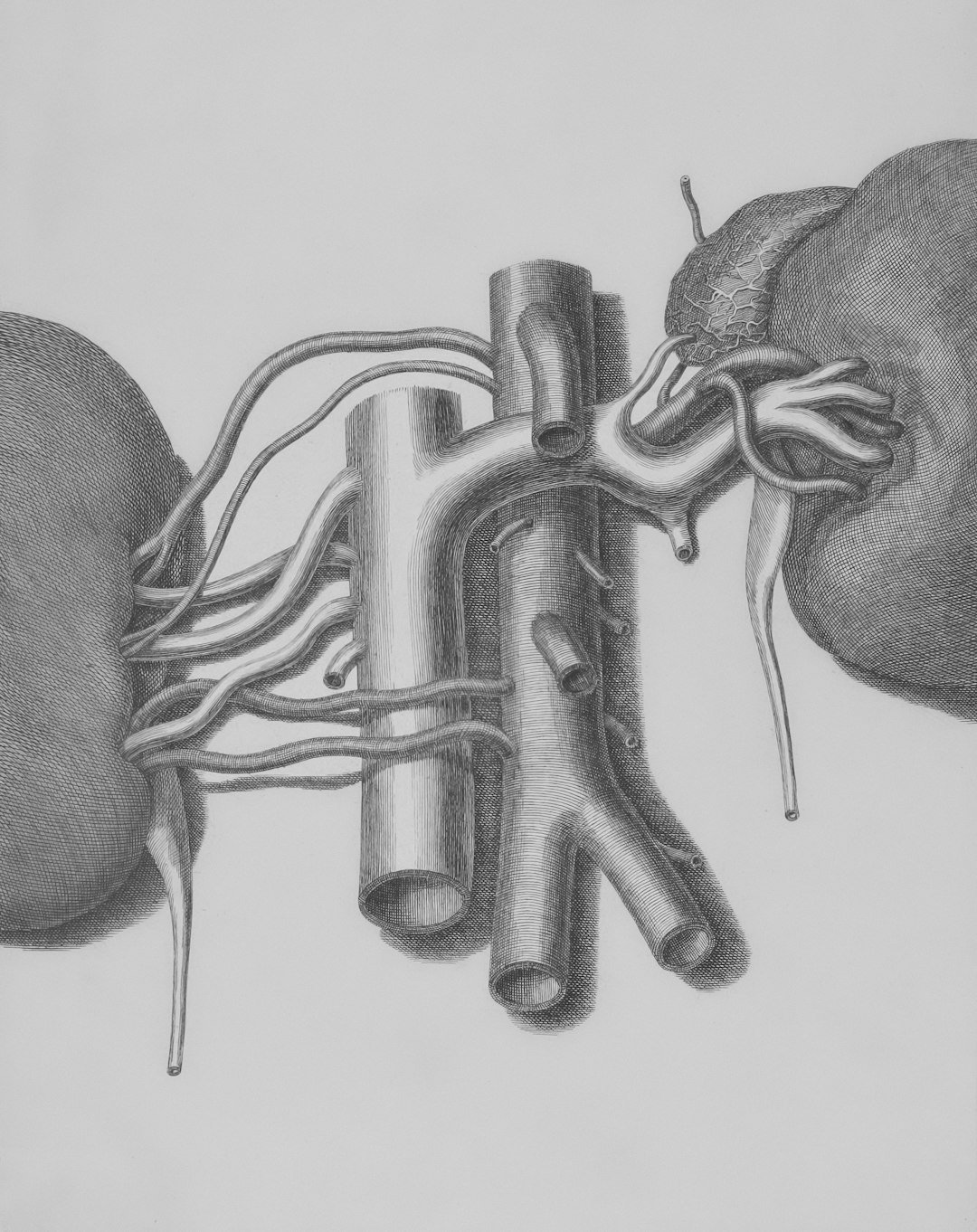
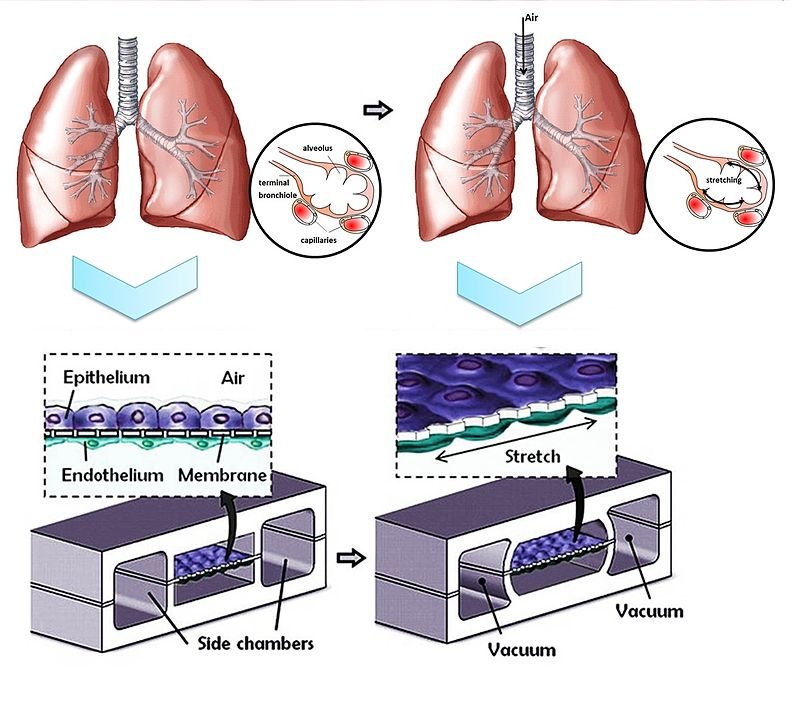
To see what true perfusion looks like today, consider kidney organoids linked to microfluidic chips, where researchers have connected lab-grown microvessels to a larger engineered channel and watched labeled dextran and even red blood cells travel into organoid tissue. In that setup, endothelial cells from the organoid reach out and form tiny anastomoses – living bridges – so that fluid moves from the main “artery” into the miniature vascular bed and onward to delicate kidney structures. It’s not a full organ in a dish, but it is authentic, directed flow across multiple scales, the kind of plumbing biology needs to grow up.
Crucially, perfusion changes cell behavior and gene programs, pushing models toward maturity that static cultures rarely achieve. For cardiology, this convergence with heart-like pumping tissues suggests a near-term future where beating mini-hearts drive their own microcirculation in vitro. That’s the promise: contraction coupled to circulation, not just motion without movement.
Mini-Lungs Join the Network
Scientists at various institutions are developing miniature lungs grown with organ-specific blood vessel networks that behave much more like those in developing human tissue. These vascularized lung organoids don’t merely look the part; they unlocked fresh insights into a rare neonatal vascular disorder that standard models had missed, a telling stress test for biological realism. The work shows that vascularization isn’t a cosmetic add-on – it’s a functional key that reveals hidden disease mechanisms.
It also hints that the same playbook can be tuned for other organs like intestines and colon, widening the toolkit for studying gut-vascular crosstalk and drug absorption. The ripple effect is obvious: once you can specify organ-type vessels, you can probe questions that were previously off-limits. That’s how basic discovery becomes translational momentum.
Why It Matters
Vascularized, pumping mini-organs change the calculus for medicine because they bridge the gulf between oversimplified cell cultures and ethically complex animal or human studies. Traditional organoids taught us a lot about early development, but their lack of flow capped their maturity and misled some drug screens. With plumbing, you can ask harder questions – how a compound alters vascular tone, how immune cells traffic, how shear stress reshapes gene expression – under human conditions. Consider three practical dividends: faster iteration on drug candidates at human-relevant doses; safer toxicity testing before first-in-human trials; and more faithful models of congenital disease where early vascular cues matter.
For patients, this could translate to better dosing, fewer side effects, and therapies tailored to a person’s own stem-cell-derived tissues. That’s not hype; it’s the natural outcome when models stop pretending and start behaving.
Beyond the Hype: Limits, Safety, and Global Context

None of this is plug-and-play, and it shouldn’t be sold that way. Reproducibility still varies between labs, and not every vascular network perfuses robustly or remains stable over long periods without meticulous support. Standardized protocols, shared reference tissues, and careful validation against clinical data are essential before anyone talks about replacing tried-and-true preclinical paths. Ethically, sourcing stem cells with clear consent, protecting donor privacy in biobanks, and avoiding unrealistic expectations for transplantation remain central guardrails.
Globally, momentum spans North America and beyond, with academic centers and startups converging on the same goal: human models that actually act human. The shared challenge is to scale these systems without sanding off the biological rough edges that make them predictive in the first place.
The Future Landscape
The next leap is integration: mini-hearts that don’t just beat but actively pressurize a vascularized network, mini-lungs that exchange gases while their vessels respond to flow, and multi-organ circuits that mimic a whole-body response to a drug. Technologies now in play – organ-on-chip microfluidics, 3D bioprinting of vessel scaffolds, and genetic switches that accelerate endothelial differentiation – are being combined into hybrid platforms built for perfusion from day one. Reviews this year emphasize co-differentiation strategies and the assembly of blood-vessel organoids as plug-in components, a modular path to consistent flow across tissues.
Add immune and nerve cells, and you get feedback loops that uncover side effects before they reach a clinic. The endgame isn’t a lab-grown transplant tomorrow; it’s a smarter, safer pipeline for therapies, informed by human biology rather than approximations. That focus on function over flash is how this field will avoid the fads that once haunted tissue engineering.
What You Can Do

If this work inspires you, there are tangible ways to help it grow responsibly. Support nonprofit biobanks and academic cores that share standardized cell lines and protocols – those common resources make breakthroughs reproducible rather than lucky. Encourage journals and funders to prioritize transparent methods and open datasets so that vascularized organoids can be audited, not just admired. Consider registering as an organ and tissue donor, which advances both transplantation and basic science training.
And when you read headlines, reward nuance: seek studies that show not only pretty images but evidence of flow, function, and validation against human data. In a field finally learning to move fluids, skepticism guided by curiosity is the best pressure gauge.

Suhail Ahmed is a passionate digital professional and nature enthusiast with over 8 years of experience in content strategy, SEO, web development, and digital operations. Alongside his freelance journey, Suhail actively contributes to nature and wildlife platforms like Discover Wildlife, where he channels his curiosity for the planet into engaging, educational storytelling.
With a strong background in managing digital ecosystems — from ecommerce stores and WordPress websites to social media and automation — Suhail merges technical precision with creative insight. His content reflects a rare balance: SEO-friendly yet deeply human, data-informed yet emotionally resonant.
Driven by a love for discovery and storytelling, Suhail believes in using digital platforms to amplify causes that matter — especially those protecting Earth’s biodiversity and inspiring sustainable living. Whether he’s managing online projects or crafting wildlife content, his goal remains the same: to inform, inspire, and leave a positive digital footprint.



