In a landmark move for reproductive biology, researchers have grown the most advanced artificial amniotic sacs yet developed in a lab raising basic questions regarding the future of pregnancy science and treatment for infertility. The sacs filled with liquid, grown from stem cells, mimic the natural protective shell that surrounds an embryo developing, providing scientists with an unprecedented glimpse of human embryonic development.
Published in Cell, the study details how scientists coaxed stem cells into forming sacs roughly 2 centimeters (0.8 inches) wide comparable to those found in a four-week-old embryo. While far from a synthetic womb, this breakthrough could revolutionize our understanding of miscarriage risks, birth defects, and even the ethical boundaries of lab-grown reproduction.
The Amniotic Sac: Nature’s First Shield for the Embryo
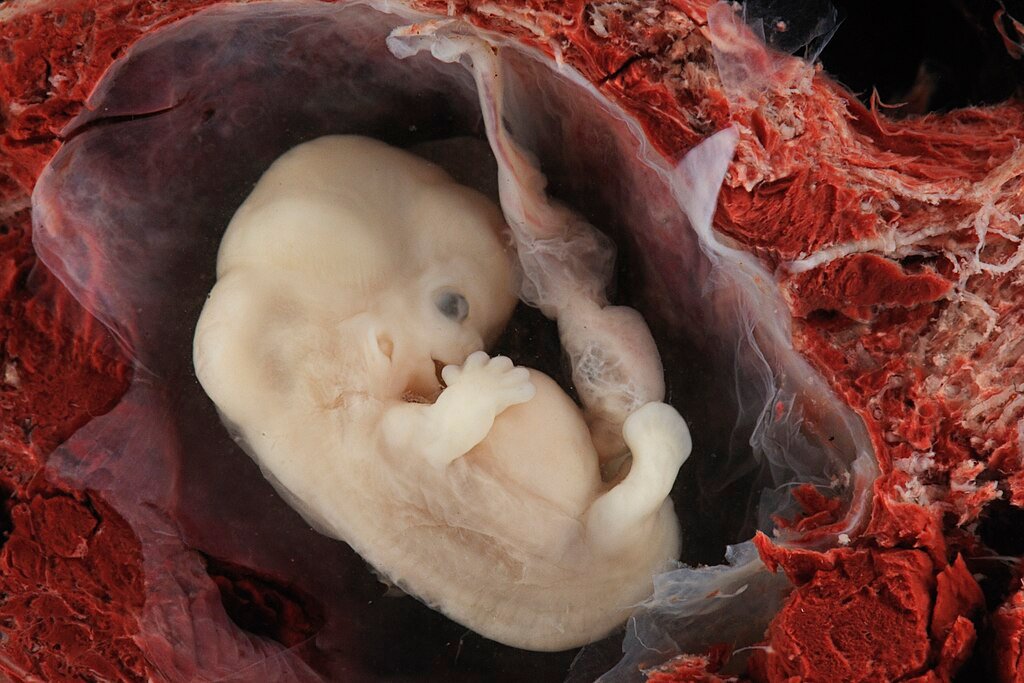
The amniotic sac is one of the first structures to develop during pregnancy a thin, watery membrane that cushions and protects the growing embryo. It serves as a biological shock absorber, helps keep the embryo at an optimal temperature, and even helps exchange nutrients. However, observing it in real time has been practically impossible owing to ethical and technological constraints.
Now, for the first time, scientists at the Francis Crick Institute in London have recreated this critical structure outside the womb. “It’s quite remarkable, the self-organization properties of these cells,” says co-author Silvia Santos, a stem-cell biologist involved in the study. The artificial sacs not only formed spontaneously but also expanded over three months, mirroring early fetal development.
How Scientists Built an Artificial Amniotic Sac
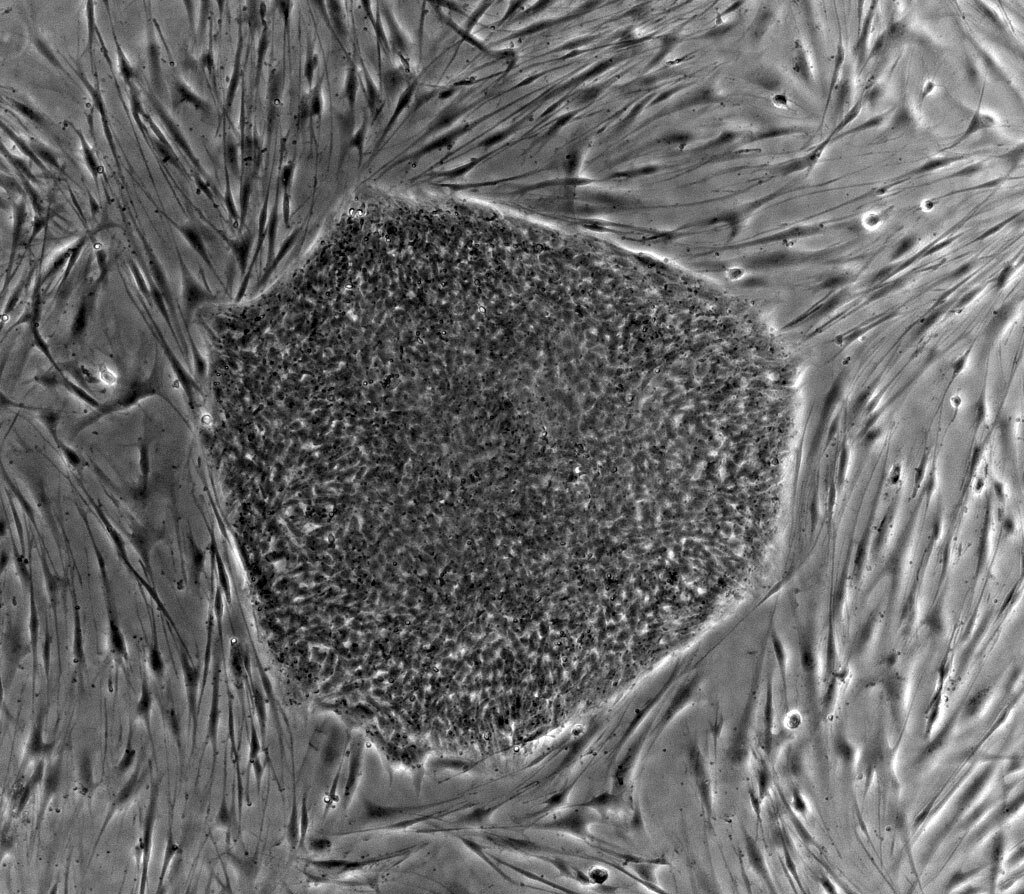
To engineer the sacs, researchers led by stem-cell biologist Borzo Gharibi used a carefully timed chemical dance. They first exposed embryonic stem cells to one signaling molecule for 24 hours, then switched to another the next day. Once transferred to a nutrient-rich culture medium, the cells self-assembled into tiny, fluid-filled structures.
Over time, these sacs grew to 2 centimeters in diameter similar to a natural four-week amniotic sac. Even more astonishing, a temporary yolk sac (which normally nourishes the embryo) appeared before vanishing after two weeks, just as it would in a real pregnancy.
A Treasure Trove of Biological Clues in Synthetic Fluid
One of the strongest benefits of this model? The chance to examine the amniotic fluid itself. When scientists took the lab-grown fluid and extracted it for analysis, they discovered it was filled with proteins and metabolites essential for fetal development many of which were similar to those identified in later-term human pregnancies.
This discovery suggests the synthetic sacs could help scientists:
- Identify early biomarkers for developmental disorders.
- Test drugs for safety during pregnancy without risking human embryos.
- Investigate why some pregnancies fail due to amniotic sac malfunctions.
Limitations: How Close Is This to a Real Womb?
Despite its promise, the model has key limitations. Janet Rossant, a developmental biologist at Toronto’s Hospital for Sick Children, cautions that the sacs only mimic very early pregnancy stages. “The impact of the amnion is in its relationship with the embryo itself,” she says. “As a free-standing amniotic sac, it doesn’t have a whole lot of significance.”
Most pregnancy complications such as preterm rupture of membranes or infections occur much later in gestation. To study those, scientists would need to develop more advanced models that replicate later developmental phases.
The Next Frontier: Embryo and Amnion Interaction
The team’s next goal? Growing even larger sacs and introducing embryo-like cells into the fluid to observe how they interact. “This would be important to gain relevant scientific insights,” says Rossant. If successful, such experiments could unlock secrets of early miscarriage or genetic abnormalities.
But this research also ventures into some fairly questionable ethical ground. Although synthetic embryos are at present impossible, stem-cell technology is moving so fast it’s making the distinction between laboratory models and real reproduction increasingly tenuous.
Could This Lead to Artificial Wombs?
Though still a long way from in vitro gestation, the research takes science closer to being able to grasp and possibly mimic the earliest moments of human development. Man-made amniotic sacs won’t be replacing normal pregnancy any time in the near future, but they may lead to:
- Safer fertility treatments.
- Better understanding of congenital diseases.
- Breakthroughs in regenerative medicine.
As scientists refine these models, society will face tough questions: How far should lab-grown reproduction go? And what does it mean for the future of human life?
For the time being, the research holds out promise not for artificial wombs, but for greater understanding of why some pregnancies end successfully, while others end in heartbreaking failure. And within reproductive science, that’s revolutionary.
Sources:

Jan loves Wildlife and Animals and is one of the founders of Animals Around The Globe. He holds an MSc in Finance & Economics and is a passionate PADI Open Water Diver. His favorite animals are Mountain Gorillas, Tigers, and Great White Sharks. He lived in South Africa, Germany, the USA, Ireland, Italy, China, and Australia. Before AATG, Jan worked for Google, Axel Springer, BMW and others.



