Imagine holding the world’s tiniest scalpel, one that can slice and rearrange the very code of life itself. That’s the power of CRISPR — a gene-editing tool that has been hailed as a miracle and, sometimes, feared as a Pandora’s box. But what happens when this scalpel slips? When CRISPR makes a mistake, the consequences can be as subtle as a single misplaced letter in a genetic sentence — or as catastrophic as a biological disaster. The truth is, gene editing is not always precise, and its mishaps can be as fascinating as they are frightening. Step into the world where science fiction meets reality, where the tiniest error could change an organism — or an entire species — forever.
The Promise and Peril of CRISPR Technology
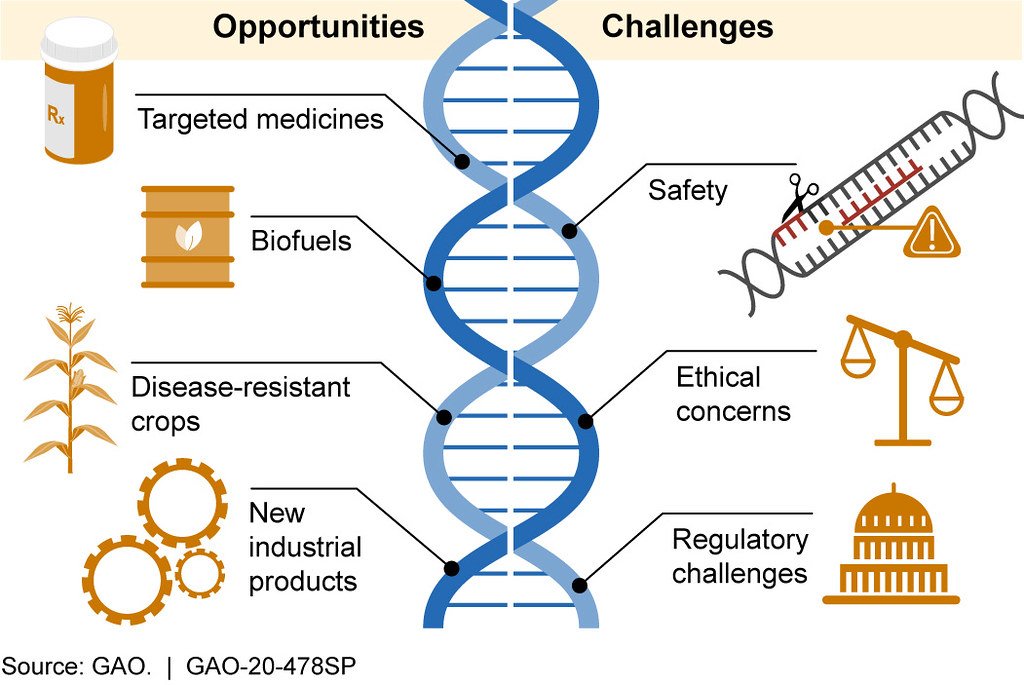
CRISPR has revolutionized the way scientists think about genetic engineering. It offers the tantalizing ability to correct disease-causing mutations, create pest-resistant crops, and even bring extinct species back to life. The technique is often compared to a pair of molecular scissors, snipping DNA at just the right spot. But every powerful tool comes with risks. When the cut isn’t perfect, or when the repair process goes awry, the results can be unpredictable. The allure of controlling evolution is matched only by the anxiety over what happens when things don’t go as planned. The double-edged sword of CRISPR reminds us that with great power comes great responsibility.
Off-Target Effects: The Hidden Dangers
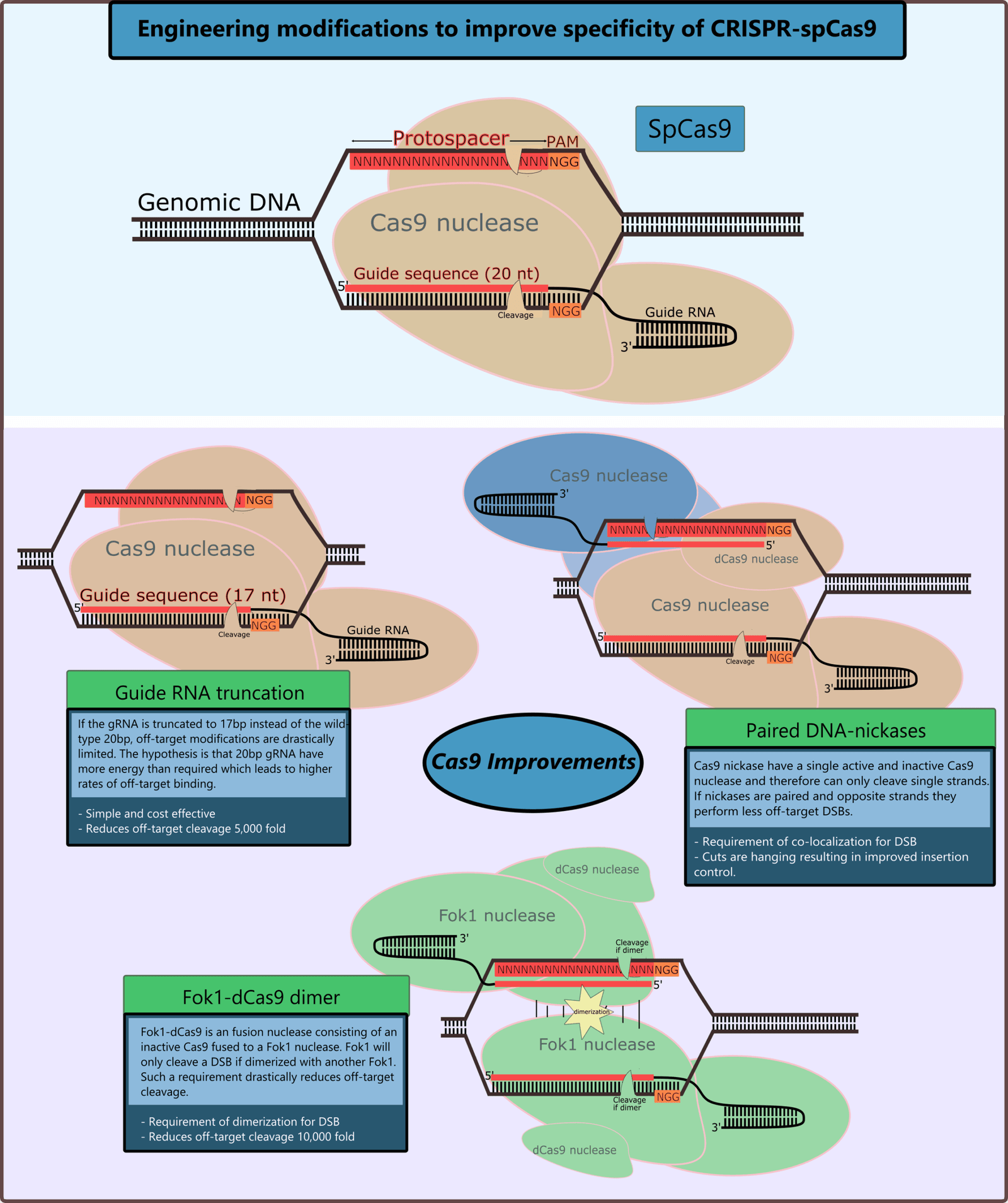
One of the most unsettling aspects of CRISPR is its propensity for off-target effects. This happens when the tool edits the wrong part of the genome, creating unintended mutations. Imagine trying to correct a typo in a book, but accidentally changing a word in a completely different chapter. These genetic blunders can lead to unexpected consequences, such as the activation of cancer genes or the disruption of essential cellular processes. Scientists work tirelessly to minimize these risks, but the reality is that no technology is foolproof. The invisible nature of off-target effects makes them especially dangerous, as they can lurk undetected until it’s too late.
Genetic Mosaicism: A Patchwork of Outcomes
CRISPR doesn’t always edit every cell in an organism the same way. Sometimes, only a subset of cells receive the intended change, while others remain untouched or are edited differently. This creates what’s known as genetic mosaicism — a patchwork of genetically distinct cells within the same individual. The results can be unpredictable, ranging from harmless oddities to serious health problems. In animal models, mosaicism has led to bizarre patterns of fur color or physical abnormalities. In humans, the effects could be far more profound, especially if edited embryos are involved. The patchwork nature of mosaicism is a reminder that biology rarely follows a script.
Unintended Consequences in Human Embryos
Editing the genes of human embryos is one of the most controversial uses of CRISPR. The dream is to eliminate genetic diseases before birth, but the reality is much messier. Even a minor mistake in editing can lead to lifelong consequences for the child, and potentially their descendants. In 2018, the world was shocked when a Chinese scientist announced the birth of CRISPR-edited babies. The scientific community quickly condemned the experiment, citing ethical concerns and a lack of oversight. The case highlighted just how little we know about the long-term effects of editing human embryos — and how high the stakes can be when things go wrong.
Immune Responses: When the Body Fights Back

CRISPR relies on proteins derived from bacteria, which can trigger immune responses in humans and animals. Sometimes, the body detects these foreign proteins and mounts an attack, undermining the gene-editing process. This can not only reduce the effectiveness of the treatment but also cause inflammation or other harmful reactions. In some cases, the immune response can be dangerous or even deadly. Researchers are now exploring ways to cloak CRISPR proteins or engineer them to be less recognizable by the immune system. The struggle between human ingenuity and nature’s defenses is as old as medicine itself.
Unexpected Genetic Rearrangements
Besides making clean cuts, CRISPR can sometimes cause unexpected rearrangements in the DNA. These include large deletions, duplications, or inversions that weren’t part of the original plan. It’s as if a book editor, instead of correcting a sentence, accidentally tears out whole pages or moves paragraphs around. These rearrangements can disrupt essential genes or regulatory regions, leading to unforeseen health problems. The unpredictability of these changes underscores the need for rigorous screening and follow-up in gene-editing experiments. They serve as a humbling reminder that the genome is a complex, interconnected system.
Gene Drives: Unleashing the Uncontrollable

Gene drives are a special application of CRISPR designed to spread certain genetic traits rapidly through populations. While the idea of eradicating malaria-carrying mosquitoes sounds heroic, the risks are enormous. If something goes wrong — say, the drive spreads to a non-target species or causes harmful ecological shifts — the consequences could be irreversible. Gene drives are like opening a floodgate: once released, they’re nearly impossible to contain. This prospect has sparked heated debates among scientists, ethicists, and environmentalists. The power to rewrite ecosystems comes with the burden of unimaginable risk.
Ethical Quagmires: Where Science Meets Moral Dilemmas
CRISPR’s potential for both good and harm has ignited fierce ethical debates. Should we use it to cure disease, enhance intelligence, or create designer babies? What about unintended consequences — who is responsible if something goes wrong? These questions don’t have easy answers, and every society grapples with them differently. Some fear a slippery slope toward eugenics, while others worry about exacerbating inequalities. The ethical quagmire is deepened by the unpredictability of gene editing, which means we’re often making decisions in the dark. The choices we make today will echo for generations to come.
Animal Studies: Lessons from the Lab
Animal experiments have played a crucial role in revealing what can go wrong with CRISPR. In mice, unintended mutations have led to tumors, birth defects, and other anomalies. In crops, edits meant to improve yield have sometimes resulted in stunted growth or unexpected sensitivities. These cases serve as cautionary tales, highlighting the importance of careful testing before applying gene editing to humans. Animal studies are a vital proving ground, but they also remind us that biological systems are notoriously unpredictable. The lessons learned in the lab often come at a cost.
Environmental Risks: Nature Strikes Back
When gene-edited organisms are released into the wild, the effects can ripple through entire ecosystems. A seemingly beneficial change — like pest resistance or faster growth — can have unforeseen impacts on predators, prey, and competitors. In one case, gene-edited salmon designed to grow faster threatened to outcompete wild populations, with unknown ecological consequences. The interconnectedness of nature means that even small changes can snowball. Environmental risks are often the hardest to predict, and once the genie is out of the bottle, it’s nearly impossible to put it back.
Regulatory Challenges: Keeping Up with Innovation
The pace of CRISPR innovation has far outstripped the ability of governments to regulate it. Laws and guidelines vary wildly from country to country, creating a patchwork of oversight. Some nations ban gene editing outright, while others encourage it for agricultural or medical research. The lack of global consensus makes it easy for risky experiments to slip through the cracks. Regulators struggle to keep up with the science, leaving society vulnerable to the consequences of mistakes. Effective oversight requires not just scientific expertise but also international cooperation and transparency.
Public Perception: Fact, Fear, and Fiction
The public’s view of CRISPR is shaped by a mix of hope, hype, and horror stories. Sensational headlines about “designer babies” or “Frankenfoods” fuel anxiety, while success stories about curing genetic diseases inspire hope. This divide makes it hard to have nuanced conversations about the real risks and benefits. Misinformation can spread quickly, leading to backlash or misguided policies. Scientists must work harder than ever to communicate the complexities of gene editing in language everyone can understand. The fate of CRISPR may depend as much on public trust as on scientific progress.
Human Error: The Weakest Link

No technology is immune to human error, and CRISPR is no exception. Mistakes can occur at any stage — from designing the genetic target, to delivering the editing components, to interpreting the results. Sometimes, a simple oversight or a miscalculation can have cascading effects. Even with the best training and intentions, humans are fallible. The margin for error in gene editing is razor-thin, making vigilance and humility essential. The possibility of a small mistake leading to a big problem is a sobering thought for every researcher.
Legal and Social Repercussions
When gene editing goes wrong, the fallout isn’t just scientific — it can be legal and social as well. Lawsuits may arise from unintended side effects or accidents, and public trust can be eroded overnight. In cases where edited organisms escape containment, the blame game can become heated. The social consequences can last for years, affecting funding, research policies, and even international relations. The legal landscape surrounding CRISPR is still evolving, and every high-profile mistake adds another layer of complexity. The stakes are high, not just for scientists, but for society as a whole.
Personal Stories: When Hopes Are Dashed
Behind every scientific mishap are real people whose lives are affected. Patients who put their faith in gene-editing treatments can be devastated when things go wrong. Families hoping for a cure may instead face new medical complications or uncertainty. The emotional toll can be enormous, turning hope into heartbreak. These personal stories are often overlooked in scientific discussions, but they are a crucial part of the picture. Gene editing is not just an abstract concept; it’s something that touches lives in deeply personal ways.
Lessons from Nature’s Own “Editing”
Interestingly, nature has been editing genes for billions of years through mutation and natural selection. Sometimes, these natural edits create new species or traits; other times, they lead to disease or extinction. CRISPR is powerful because it lets us mimic — and sometimes outpace — nature’s methods. But nature’s mistakes can be devastating, and ours can be, too. Looking to the natural world for guidance can help us understand both the promise and the peril of our own interventions. The line between natural and artificial mistakes is often blurrier than we think.
The Race for Safer Solutions

Scientists around the world are racing to make CRISPR safer and more precise. New techniques, such as base editing and prime editing, promise to reduce the risk of mistakes. Improved screening methods can help catch errors before they cause harm. The drive for safer gene editing is fueled by both scientific curiosity and a sense of moral duty. Every advance brings us closer to realizing the full potential of CRISPR — but also highlights how much we still have to learn. The quest for perfection is never-ending, but each step forward matters.
The Importance of Transparency and Dialogue
Open communication between scientists, policymakers, and the public is crucial for responsible gene editing. Transparency about risks, failures, and uncertainties builds trust and helps prevent disastrous mistakes. Public dialogue ensures that diverse perspectives are considered, especially when the stakes are so high. When decisions are made behind closed doors, the potential for backlash and mistrust grows. A culture of openness can help society navigate the ethical and practical challenges of CRISPR. Honest conversations may be our best safeguard against the unknown.
Looking to the Future: Balancing Hope and Caution
As gene editing continues to advance, the balance between hope and caution becomes ever more important. The potential to cure diseases and improve lives is enormous, but so are the risks of unintended consequences. The challenge is to move forward bravely, but not blindly. Scientists, regulators, and the public must work together to ensure that progress does not come at the cost of safety or ethics. The future of CRISPR will be shaped by the choices we make today — and by our willingness to learn from our mistakes.




