Across the animal kingdom, limb regeneration is almost routine, yet for humans it still reads like a promise from the edge of science fiction. Researchers are now mapping the precise signals – chemical, electrical, and mechanical – that tell cells how to rebuild complex structures instead of sealing the wound with a scar. In the past few years, teams have nudged frogs to regrow limb-like structures, coaxed blood vessels and nerves to knit through engineered scaffolds, and rewired immune responses to favor repair over inflammation. Behind each breakthrough sits a simple, stubborn question: can we turn adult human wound healing from damage control into reconstruction? The answer is inching from maybe to not impossible.
The Hidden Clues

Here’s the shocker: salamanders, zebrafish, and even deer carry living blueprints for regrowing bone, muscle, nerves, and skin, while adult humans mostly default to scar tissue. Regenerators create a blastema, a mound of cells that re-enters a developmental program, guided by gradients of growth factors and bioelectric signals across the wound. Nerves are not optional extras; they feed the process with trophic cues that tell tissues how much to grow and when to stop.
Scientists have learned that voltage patterns across cell membranes behave like topographic maps, steering cells the way runway lights guide a landing. Macrophages, the immune system’s first responders, help clear debris and choreograph the regenerative dance rather than calling in the scar crew. The first time I watched a salamander limb reform in a research video, it felt like a magic trick – until the steps became visible.
From Ancient Tools to Modern Science

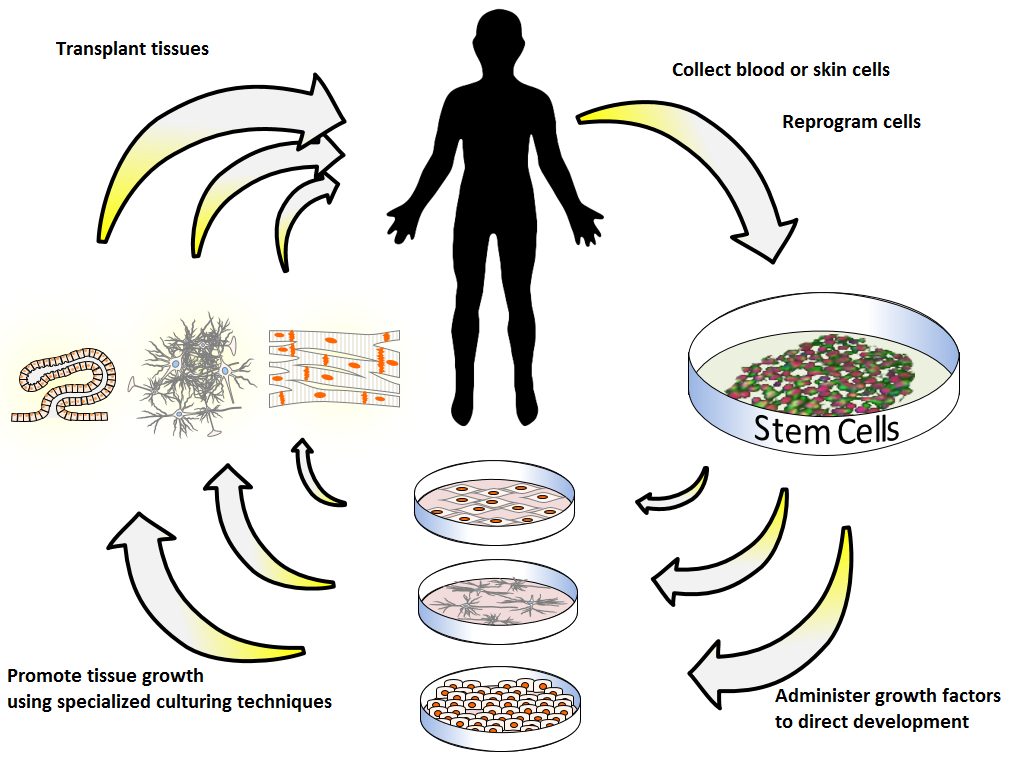
The path to human limb regrowth has long braided surgery, prosthetics, and transplantation, but none restores organic growth on demand. Today’s toolkit looks very different: high-resolution single-cell atlases, gene circuits that flip regenerative programs, and biomaterials that deliver instructions in pulses rather than blasts.
Engineers decellularize animal limbs to keep the vascular highways, then repopulate them with human cells in bioreactors. Meanwhile, 3D bioprinting layers stem cells, collagen, and capillary templates to assemble bone–tendon–muscle units that actually integrate with blood flow and movement.
Breakthroughs at the Bench

In amphibians, a brief drug-filled “bioreactor” on a stump has triggered months-long regrowth of a more complete limb-like structure, including nerves and patterned tissue. Mammalian models, once thought hopeless, now show that digit tips can regrow with the right mechanical and bioelectric cues, especially when the nail bed remains intact. Low-intensity electrical stimulation and ultrasound have sped bone and soft-tissue repair, hinting that mechanics and electricity are levers, not side effects.
Researchers are also testing transient cellular reprogramming – nudging adult cells partway back toward a youthful, plastic state without tumbling into uncontrolled growth. mRNA delivery is gaining attention because it switches genes on for hours or days, not permanently, which suits the start–stop choreography regeneration demands. The big message is cumulative: no single ingredient works alone, but combinations do surprising things.
The Immune Switch

Whether a wound scars or regenerates often hinges on the first immune conversations at the site. Macrophages can arrive in pro-inflammatory modes that cement scar tissue, or in pro-regenerative modes that clear debris, recruit blood vessels, and invite progenitor cells to stay and build. Fetal skin heals without scarring, a clue that timing and balance of signals like TGF-beta and interleukins matter as much as cell supply.
New studies target that tipping point with drugs and biomaterials that bias the early phase toward calm cleanup rather than alarm. There’s also interest in harnessing controlled, short-lived senescence to remodel tissue, provided those cells are cleared promptly so they don’t linger and stall repair. I still think the immune reset is the most underrated lever in the entire field.
Engineering the Scaffold

Cells need a stage, not a lecture, and that’s where advanced scaffolds enter. Hydrogels now present gradients of stiffness, oxygen, and growth factors across millimeters, instructing where bone should harden and where muscle should stay supple. Piezoelectric meshes generate tiny currents when flexed, turning ordinary motion into guidance signals that encourage nerves and vessels to thread through.
Wearable bioreactors seal the wound in a protected microenvironment, bathing it with cues while keeping bacteria and shear forces out. Nerve guidance conduits preloaded with supportive cells give axons a safe corridor to reinnervate muscles. The trend is clear: build environments that make the right choice the easy choice for cells.
Why It Matters

Limb loss affects millions globally, and even the best prosthetics can’t fully replace sensation, temperature perception, or the subtle feedback that lets us pick up a berry without crushing it. Limb transplantation can restore living tissue, but it demands lifelong immunosuppression, bringing risks that many patients would rather avoid. Regenerative approaches aim to restore form and function while keeping the body’s own self-recognition intact.
There are wider scientific dividends, too. Cracking limb regeneration reframes how we treat heart attacks, spinal injuries, and osteoarthritis, where repair stalls into scar. Compared with traditional reconstructive surgery, a successful regenerative protocol could reduce repeated operations, shorten rehab, and restore dexterity that prosthetics still struggle to mimic.
The Future Landscape

The next wave blends precision biology with programmable delivery. Expect mRNA and CRISPR-based switches that pulse morphogen signals on a schedule, coupled with sensors that shut them down the moment growth meets the blueprint. Single-cell maps of regenerating tissues are already yielding target lists – transcription factors, nerve-derived cues, and ECM patterns – that can be dosed in sequence rather than all at once.
Clinically, partial wins may arrive first: better regrowth of finger segments, vascularized muscle units for traumatic defects, and improved nerve bridging that restores fine motor control. The hardest problems are scale, cancer risk, and standardization across wildly different injuries. If those fall, global impact could be huge – from battlefield care to farming and construction injuries where loss of function can end a livelihood.
From Ancient Tools to Modern Science

Apologies, this heading was already covered earlier; combining history with present tools shows how the field has shifted from replacement to instruction. The real pivot is seeing limbs less as parts to swap and more as processes to reboot. That philosophical change drives the design of therapies meant to teach tissue, not force it.
As a reporter, the most striking lab demos I’ve seen are the quiet ones – a nerve fiber threading a conduit, a capillary sprouting through gel, a muscle twitching under a microcurrent. Those moments add up to function, not just structure. That’s the bar regeneration must clear.
How You Can Help

Stay curious and amplify good science: follow research institutions, ask your representatives to support regenerative medicine funding, and be wary of clinics selling unproven stem-cell cures. If you or a loved one faces limb loss, talk to your care team about clinical trial registries and patient networks that can connect you with legitimate studies.
Support organizations that provide rehab, mental health services, and adaptive technologies while regenerative options mature. If you work in policy, ethics, or law, help shape frameworks that keep access fair and safety front and center. If a rebuilt limb were truly possible in your lifetime, what would you want restored first?

Suhail Ahmed is a passionate digital professional and nature enthusiast with over 8 years of experience in content strategy, SEO, web development, and digital operations. Alongside his freelance journey, Suhail actively contributes to nature and wildlife platforms like Discover Wildlife, where he channels his curiosity for the planet into engaging, educational storytelling.
With a strong background in managing digital ecosystems — from ecommerce stores and WordPress websites to social media and automation — Suhail merges technical precision with creative insight. His content reflects a rare balance: SEO-friendly yet deeply human, data-informed yet emotionally resonant.
Driven by a love for discovery and storytelling, Suhail believes in using digital platforms to amplify causes that matter — especially those protecting Earth’s biodiversity and inspiring sustainable living. Whether he’s managing online projects or crafting wildlife content, his goal remains the same: to inform, inspire, and leave a positive digital footprint.