A grand, unsettling question is quietly moving from science fiction into lab conversations: could we rewrite human DNA so viruses simply can’t take hold? The idea landed in my inbox after yet another season of respiratory bugs, and it felt both audacious and strangely inevitable. We’ve learned to design vaccines in weeks and read genomes in hours, but viruses still dart through our defenses like rain through a picket fence. Now, some biologists suggest a bolder line of defense – changing the fence itself. If the cell’s rules were different, they argue, the viral playbook might fail before the first move.
The Hidden Clues
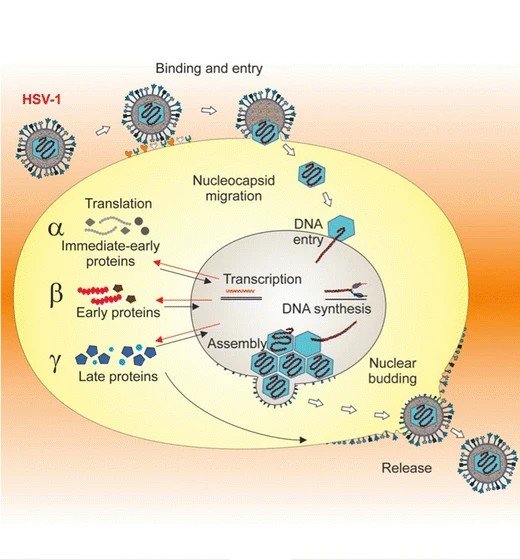
Here’s a startling truth to start with: viruses don’t really live so much as they borrow life from us, hijacking our cellular machinery line by line. That dependency is a weakness, because many viruses lean on the same handful of cellular doors and switches. If we could reinforce or reroute those shared points – receptors, translation factors, certain sugar decorations on proteins – then lots of viruses might slip and fall at the threshold.
Natural experiments already exist in the population. People with a particular change in a gene called CCR5 can be highly protected against one strain of HIV, a reminder that host genetics can redraw the battlefield. I still remember watching a virologist show me a dish of cells where infection simply fizzled because one key door was missing; it felt like seeing a magic trick explained.
From Ancient Tools to Modern Science
Our ancestors blunted viruses with isolation, luck, and the occasional plant remedy; the modern era added vaccines and a small but vital class of antiviral drugs. These tools work, but they’re reactive and specific – one virus, one countermeasure, a race that never really ends. Gene editing promises something different: altering the host so the same vulnerability can’t be exploited again and again.
Researchers have edited human cells to remove viral entry points in the lab, making them resistant to particular pathogens without obvious damage to basic cell functions. Others are exploring tweaks to innate immune circuits so cells sense and shut down viral replication sooner. It’s a leap from culture dishes to whole people, but the outlines of a strategy are there.
Inside the Virus Playbook
Viruses come in wildly different flavors – DNA and RNA, enveloped and naked, tiny minimalist genomes and sprawling giants – but they converge on a few steps: attachment, entry, translation, replication, assembly, and escape. Knock out one step and the entire operation can collapse like a scaffold missing a crossbar. That’s why the dream of broad resistance isn’t entirely naive.
The enemy, though, is evolution. Many viruses mutate quickly, rerouting to a backup receptor or shifting a protein surface to dodge our edits. Any plan aiming at “all viruses” has to be resilient to this shape-shifting dance, not just clever for a season.
The Genetic Code Gambit
A more radical idea targets the very language of life: the genetic code. In bacteria, scientists have recoded genomes so that certain three-letter “words” no longer mean what invading viruses expect, creating cells that are effectively unreadable to many phages. This genetic firewall works because viral genes arrive speaking the old dialect, and the cell no longer recognizes it.
Translating that feat to human cells would be a moonshot. Our genome is vast, our development exquisitely tuned, and our proteins woven into countless interactions that can’t be casually rewritten. Still, projects aimed at building mammalian cells with a streamlined or slightly altered code are inching forward, testing whether a safe, virus-resistant “dialect” might exist for complex cells. If even a limited recoding proved viable in certain tissues, it could give us targeted pockets of resistance.
Why It Matters
The stakes are not theoretical. Viral diseases continue to rack up hospitalizations, long-term complications, and economic shocks that ripple for years. Traditional countermeasures are essential but brittle: a new strain, a missed vaccination window, or a supply chain hiccup, and the dominoes tilt the wrong way.
Engineering host resistance could change the rhythm of outbreaks, tilting the field before viruses find footing. It might shorten epidemics, protect the most vulnerable, and reduce the need for blanket restrictions that hit livelihoods. Compared with chasing each virus separately, a host-focused shield promises compounding returns, even if it never becomes truly universal.
Global Perspectives

Any path to engineered resistance will run through ethics boards, regulators, and public trust. Editing cells in an adult to treat disease sits in a different moral neighborhood than altering embryos, and most countries keep firm lines around the latter. The world has already seen how a single reckless experiment can set progress back by years, sowing fear where careful science needed oxygen.
Equity is a second gate. A future where wealthy health systems deploy genetic resistance while others wait would deepen existing divides. If this technology advances, global partnerships and transparent governance will matter as much as lab breakthroughs, or the benefits will pool where they are least needed.
The Future Landscape
Near-term efforts will likely focus on narrow but powerful targets: editing receptors in specific cell types, hardening translation factors that multiple viruses rely on, and installing genetic “tripwires” that sense foreign RNA and halt replication. Delivery will be the practical bottleneck, since getting safe, precise edits into the right tissues remains challenging. Even incremental wins – say, lungs that are much harder for respiratory viruses to colonize – could transform seasonal disease.
Further out lies partial recoding of human cell lines for therapies, implants, or biomanufacturing that can’t be hijacked by viruses. Completely rebooting the human genetic code is far beyond today’s tools and probably a poor bargain with biology, but strategic edits could still build meaningful firebreaks. The arc here looks less like a single invention and more like a layered defense built piece by piece.
Real-World Trials and Ethical Fault Lines
Proof will come from cautious clinical pilots where the benefit is clear and the genetics are straightforward – fortifying immune cells for chronic viral infections, for instance, or protecting transplant tissues that are especially vulnerable. Safety monitoring will need to be relentless, looking for subtle trade-offs like increased susceptibility to other pathogens or unintended immune misfires. Biology rarely offers free lunches, and host defenses are a web; tug one thread, another moves.
I think back to the first time I handled a genome editing kit in a classroom: simple pipettes, bright labels, and a sense that power had gotten portable. That feeling is both thrilling and sobering today. Guardrails – technical fail-safes, independent oversight, and open data – aren’t distractions from innovation; they’re the scaffolding that lets it climb.
How You Can Engage

Curiosity is the first step. Follow credible science outlets, ask your local representatives how they weigh gene-editing proposals, and look for community forums where researchers explain their work in plain language. If you’re able, support organizations that expand access to vaccines and antivirals now, because better present-day defenses buy time for careful, long-horizon research.
Students and educators can push for curriculum that demystifies genomics, while patients can join registries and trials that advance ethical, transparent studies. And all of us can practice a small, stubborn skepticism – demanding evidence without defaulting to fear. In the end, the question isn’t whether we could edit our DNA to resist every virus, but how far we should go to resist the next one – what would you choose?

Suhail Ahmed is a passionate digital professional and nature enthusiast with over 8 years of experience in content strategy, SEO, web development, and digital operations. Alongside his freelance journey, Suhail actively contributes to nature and wildlife platforms like Discover Wildlife, where he channels his curiosity for the planet into engaging, educational storytelling.
With a strong background in managing digital ecosystems — from ecommerce stores and WordPress websites to social media and automation — Suhail merges technical precision with creative insight. His content reflects a rare balance: SEO-friendly yet deeply human, data-informed yet emotionally resonant.
Driven by a love for discovery and storytelling, Suhail believes in using digital platforms to amplify causes that matter — especially those protecting Earth’s biodiversity and inspiring sustainable living. Whether he’s managing online projects or crafting wildlife content, his goal remains the same: to inform, inspire, and leave a positive digital footprint.