When we picture extraterrestrial life, most of us imagine something fundamentally similar to Earth’s creatures. But what if we’re thinking too small? What if alien life forms are constructed from entirely different building blocks than the carbon-based chemistry we know and love? The universe is vast and strange, and the possibilities for alternative life may be more diverse than our wildest science fiction dreams. Some scientists suggest that silicon, with its chemical versatility, could serve as a backbone for life in ways strikingly similar to carbon. Others wonder if exotic solvents like ammonia or even methane could replace water as the medium of biology. Then there are theories proposing plasma-based or even machine-like life forms that stretch the definition of “alive” altogether. By exploring these radical possibilities, researchers aren’t just expanding the search for aliens—they’re forcing us to reconsider what life itself really means. After all, if we only look for Earth-like organisms, we might miss entirely new forms of existence hiding in plain sight.
The Dominance of Carbon on Earth – But Is It Universal?

The great versatility of the carbon atom makes it the element most likely to provide solutions, even exotic solutions, to the problems of survival on other planets. Here on our planet, every single living thing we’ve discovered shares the same fundamental chemistry. All known living things have a carbon-based structure and system, with all the main molecules used in organic processes built on skeletons of carbon and hydrogen.
But this doesn’t mean carbon is the only game in town across the cosmos. There was only a remote possibility that non-carbon life forms could exist with genetic information systems capable of self-replication and the ability to evolve and adapt. However, that remote possibility is exactly what makes the search for alternative biochemistries so thrilling. The universe might be teeming with life forms that would seem utterly alien to us simply because they’re built from different atomic ingredients.
Silicon – The Most Obvious Alternative That Doesn’t Quite Work

The element silicon has been much discussed as a hypothetical alternative to carbon. Silicon is in the same group as carbon on the periodic table and, like carbon, it is tetravalent. This similarity has made silicon the poster child for alternative biochemistry in countless science fiction stories. Carbon and silicon are chemically very similar in that silicon atoms can also each form bonds with up to four other atoms simultaneously.
However, reality is more complicated than fiction. When carbon oxidizes it becomes the gas carbon dioxide; silicon oxidizes to the solid silicon dioxide, called silica. The fact that silicon oxidizes to a solid is one basic reason as to why it cannot support life. Additionally, in no environment is a life based primarily around silicon chemistry a plausible option. In a water-rich environment silicon’s chemical capacity is highly limited due to ubiquitous silica formation. Silicon just doesn’t have the chemical flexibility that life seems to require.
The Arsenic Controversy – A Glimpse Into Alternative Phosphorus
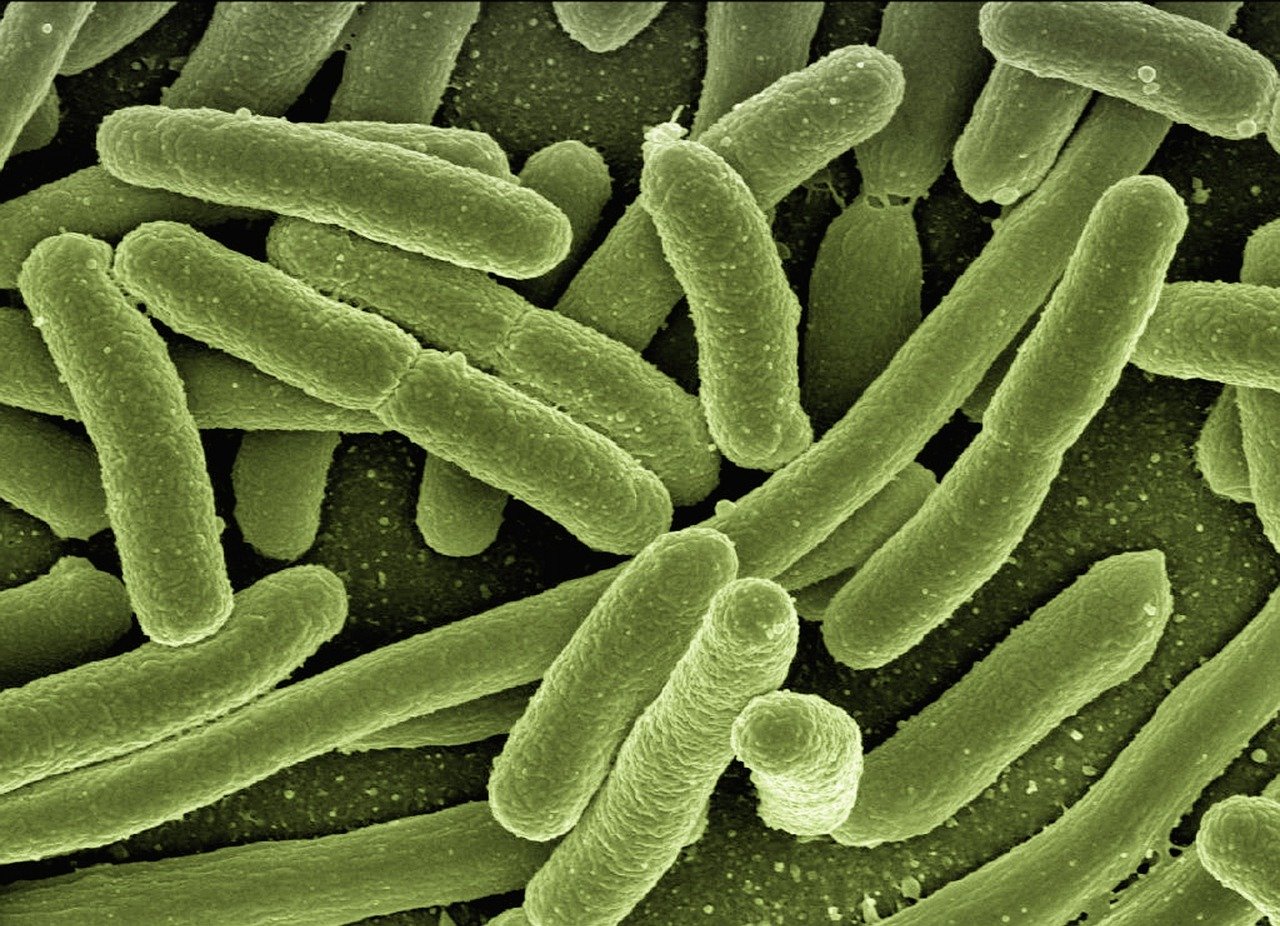
A 2010 geomicrobiology study, supported in part by NASA, postulated that a bacterium, named GFAJ-1, collected in the sediments of Mono Lake in eastern California, can employ ‘arsenic DNA’ when cultured without phosphorus. They proposed that the bacterium may employ high levels of poly-β-hydroxybutyrate or other means to reduce the effective concentration of water and stabilize its arsenate esters.
This claim was heavily criticized almost immediately after publication for the perceived lack of appropriate controls. While the arsenic-based life claim was eventually debunked, it highlighted something fascinating about alternative biochemistry. Arsenic, bismuth and antimony should be able to replace phosphorus because they’re all in the same group. It’s plausible that a DNA like molecule could form with arsenic instead of phosphorus as arsenic is directly under phosphorus on the periodic table, meaning it has the same number of valence electrons, which is important in molecular bonding.
Boron and Nitrogen – The Dynamic Duo Nobody Talks About
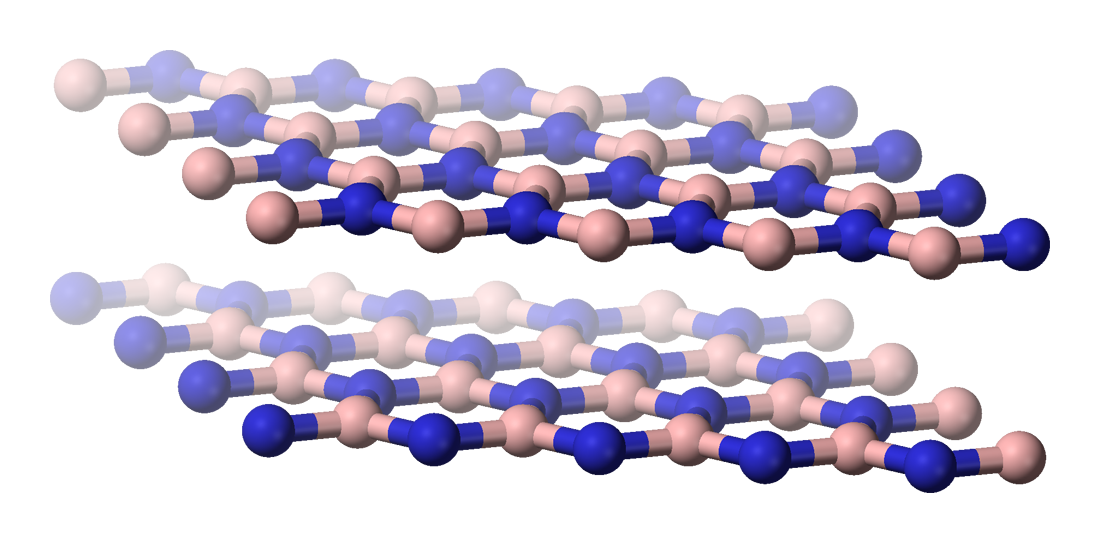
Boron is even more versatile than carbon, and while its simplest compounds, boranes, are extremely reactive in the presence of oxygen, they’d be more stable in a reducing atmosphere. A combination of alternating boron and nitrogen closely resembles carbon, and can form analogues of hydrocarbons such as ethane, methane and benzene.
This boron-nitrogen chemistry represents one of the most intriguing possibilities for alternative life. Boranes are dangerously explosive in Earth’s atmosphere, but would be more stable in a reducing atmosphere. However, boron’s low cosmic abundance makes it less likely as a base for life than carbon. In worlds with different atmospheric compositions, particularly those lacking oxygen, boron-nitrogen biochemistry could flourish where carbon-based life would struggle to survive.
Sulfur and Phosphorus – The Chain Makers
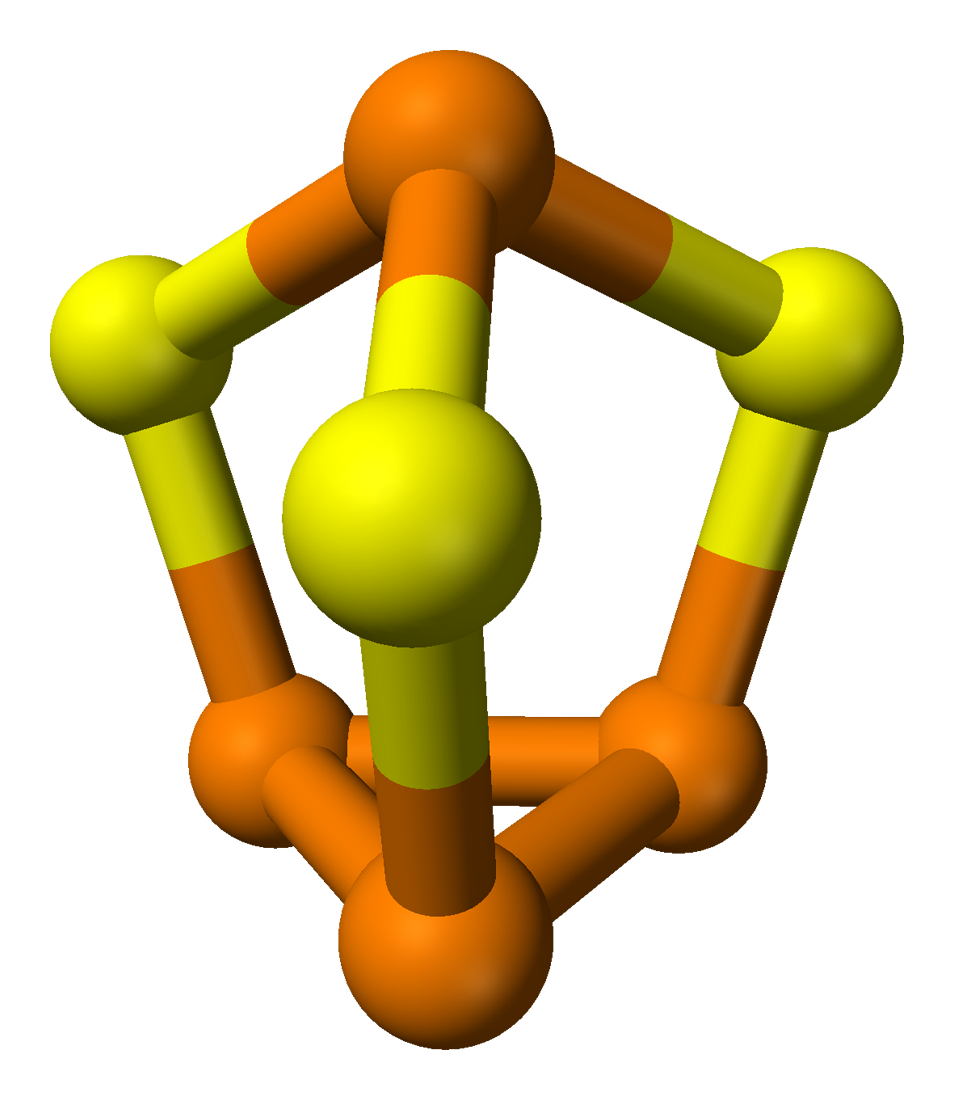
Sulfur is also able to form long-chain molecules, but suffers from the same high-reactivity problems as phosphorus and silanes. The biological use of sulfur as an alternative to carbon is purely hypothetical, especially because sulfur usually forms only linear chains rather than branched ones. Despite these limitations, both phosphorus and sulfur are able to form long linear chains, but not branched ones, and they need a reducing atmosphere to be stable.
While linear chains might seem limiting compared to carbon’s branching abilities, we shouldn’t underestimate what evolution might accomplish with such building blocks. Life has a remarkable ability to work with whatever materials are available, and perhaps in certain environments, linear polymer chains could serve as the backbone for entirely different forms of biological organization.
Fluorine Chemistry – The Forbidden Element

Life on Earth is known to rarely make fluorinated carbon compounds, as compared to other halocarbons. The exclusion of the C–F bond stems from the unique physico-chemical properties of fluorine, predominantly its extreme electronegativity and strong hydration shell. Life on Earth never makes compounds containing the N–F and S–F bonds, nor fully fluorinated non-carbon compounds. S–F and N–F bonds are not even made as metabolic intermediates and are likely to be universally excluded by life, no matter its biochemistry. In fact, life on Earth very rarely uses F chemistry.
But here’s where it gets interesting for alien biochemistry. A speculative evolutionary application of the C–F bond stability in an alien biochemistry could include its role as a specialized mimic of a C–H bond. Fluorine is the closest atom in size to hydrogen atom, and due to its relatively small size and very strong bond to carbon, fluorine can substitute hydrogen atoms in virtually every type of organic molecule. The ability of fluorine to “cap” carbon atoms could provide a unique advantage for life in planetary environments where water or hydrogen atoms in general remain scarce.
Alternative Solvents – Beyond Water’s Reign

Hypothetical alternatives to water include ammonia, which, like water, is a polar molecule, and cosmically abundant; and non-polar hydrocarbon solvents such as methane and ethane, which are known to exist in liquid form on the surface of Titan. Ammonia and ammonia–water mixtures remain liquid at temperatures far below the freezing point of pure water, so such biochemistries might be well suited to planets and moons orbiting outside the water-based habitability zone. Such conditions could exist, for example, under the surface of Saturn’s largest moon Titan.
Isaac Asimov, the biochemist and science fiction writer, reportedly suggested that poly-lipids could form a substitute for proteins in a non-polar solvent such as methane. Water is more chemically reactive and can break down large organic molecules through hydrolysis. A life-form which solvent was a hydrocarbon would not face the threat of its biomolecules being destroyed in this way.
The Extreme Environments – Plasma and Energy-Based Life

Feinberg and Shapiro have suggested that molten silicate rock could serve as a liquid medium for organisms with a chemistry based on silicon, oxygen, and other elements such as aluminium. But what if we think even more radically? In stars or ionospheres, self-organizing plasmoids could support life with electromagnetic flows replacing molecular bonds.
Hypothetical life could exist in superconducting lattices or Bose–Einstein condensates, where exchange occurs via quantum coherence rather than chemical reactions. Wherever sustained, ordered matter–energy transformations exist with replication, life may emerge – even in silicon deserts, sulfur seas, or plasma vortices. These concepts push the boundaries of what we consider “life” into realms that are more about organized energy patterns than traditional chemistry.
The Chirality Question – Mirror Image Life

Perhaps the least unusual alternative biochemistry would be one with differing chirality of its biomolecules. In known Earth-based life, amino acids are almost universally of the L form and sugars are of the D form. Molecules using D amino acids or L sugars may be possible; molecules of such a chirality, however, would be incompatible with organisms using the opposing chirality molecules.
Even with a biochemistry very similar to our own, there are still many possibilities for organic molecules through chirality, the orientation in space of molecules. Sugars and amino acids are asymmetrical, and the two specular forms have the same chemical properties. Terran life uses L amino acids and D sugars. Alien organisms with D amino acids could digest L sugars in the same way we do with D sugars, which they couldn’t digest. This creates the fascinating possibility of mirror-image life that’s chemically identical to ours but completely incompatible.
Metal-Based and High-Temperature Life
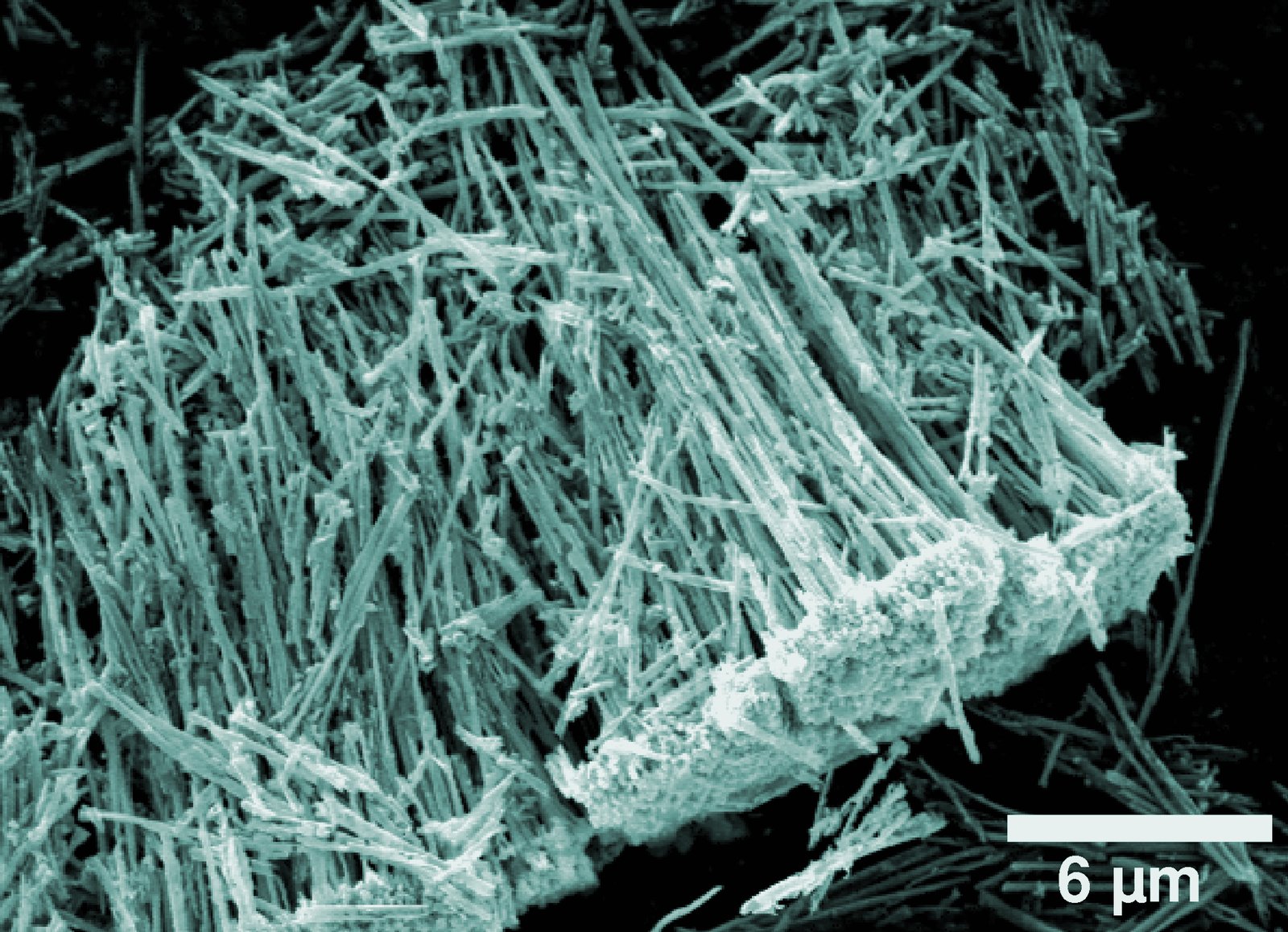
Less convincing substitutes for carbon can be metal oxides based on magnesium, aluminium, iron or titanium, which can form stable nanotubes and diamond-like crystals at high temperatures. Silicon is inert at moderate temperatures in the environments in which life as we know it exists, leading to an idea that silicon based life could perhaps only exist at very high temperatures; these potential theoretical organisms have been called “lavolobes” and “magmobes.” Unfortunately, at such very high temperatures the chemistry would be very different, and such a theoretical life form would not be something which we could encounter.
These high-temperature life forms represent perhaps the most alien concept of all. Imagine creatures that thrive in volcanic vents or in the molten interiors of planets, where the very notion of solid biomolecules breaks down entirely. Such beings might exist as organized patterns in flowing metal or as crystalline structures that grow and reproduce through geological processes.
The Laboratory Evidence – Making the Impossible Possible
Scientists have for the first time shown that nature can evolve to incorporate silicon into carbon-based molecules, the building blocks of life on Earth. “My feeling is that if a human being can coax life to build bonds between silicon and carbon, nature can do it too.” After analyzing cytochrome c’s structure, researchers suspected that only a few mutations might greatly enhance the enzyme’s catalytic activity. Indeed, only three rounds of mutations were enough to turn this protein into a catalyst that could generate carbon-silicon bonds more than 15 times more efficiently than the best synthetic techniques currently available. The mutant enzyme could generate at least 20 different organo-silicon compounds.
This breakthrough demonstrates that the boundary between “possible” and “impossible” biochemistry isn’t as solid as we once thought. “The biggest surprise from this work is how easy it was to get new functions out of biology, new functions perhaps never selected for in the natural world that are still useful to human beings. The biological world always seems poised to innovate.”
Conclusion

The question of whether alien life could be made of something other than carbon opens up a universe of possibilities that challenges our Earth-centric assumptions about biology. While carbon remains uniquely suited for complex biochemistry in water-based environments, the cosmos offers conditions so varied and extreme that alternative chemistries become not just possible, but perhaps inevitable.
From silicon-based creatures thriving in molten rock to plasma beings organizing themselves through electromagnetic forces, from mirror-image organisms that could never share a meal with us to boron-nitrogen life forms flourishing in reducing atmospheres – the universe might be far stranger and more wonderfully diverse than we’ve dared to imagine.
As we continue to discover exoplanets with conditions unlike anything on Earth, perhaps it’s time to expand our definition of what life can be. After all, in a universe this vast and varied, wouldn’t it be more surprising if Earth’s carbon-based chemistry was the only solution to the puzzle of existence? What alien chemistry might be writing its own story of life among the stars right now?

Hi, I’m Andrew, and I come from India. Experienced content specialist with a passion for writing. My forte includes health and wellness, Travel, Animals, and Nature. A nature nomad, I am obsessed with mountains and love high-altitude trekking. I have been on several Himalayan treks in India including the Everest Base Camp in Nepal, a profound experience.




