Deep beneath the rolling hills of France, in the damp, dark corridors of ancient limestone caves, a scientific revolution is quietly brewing. While tourists marvel at stalactites and underground formations, researchers have uncovered something far more extraordinary: microscopic organisms with the potential to transform how we heal broken bones and treat devastating bone infections. These cave-dwelling bacteria, isolated from the pristine environments of French caverns, aren’t just surviving in some of Earth’s most extreme conditions—they’re creating calcium-based minerals that could one day become the foundation of revolutionary bone repair treatments.
The Hidden World Beneath Our Feet

Cave ecosystems represent one of the most pristine and ancient environments on Earth, containing unique microbial communities that have evolved in complete darkness for millions of years. The microenvironments in caves are characterized by high humidity, constant temperatures, and air rich in natural antibiotics produced by fungal spores. These conditions create a perfect laboratory where bacteria have developed extraordinary survival mechanisms, including the ability to precipitate calcium carbonate—the very same material that forms the mineral component of our bones. French caves, in particular, have become treasure troves for microbiologists seeking these remarkable organisms. The cave air is naturally free from germs, dust particles, and allergens, containing beneficial calcium, magnesium, and iodine ions. Imagine discovering that the solution to your grandmother’s hip fracture might literally be hiding in the caves where ancient humans once sheltered from ice ages. The Cro-Magnon people created their extraordinary paintings on cave walls in France and Spain over 10,000 years ago, but they had no idea they were sharing their sanctuary with microscopic bone-builders.
Nature’s Master Chemists at Work
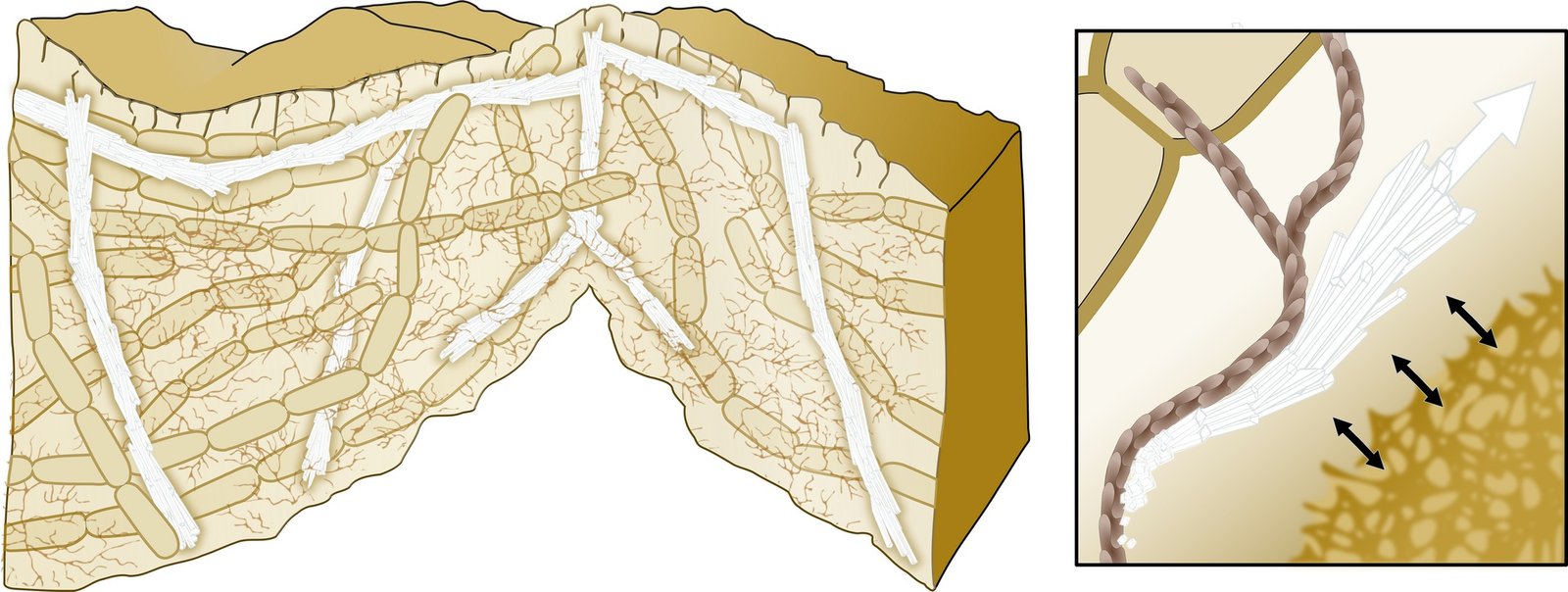
Cave bacteria isolated from Hungarian caves produce amorphous calcium carbonate (ACC), with their extracellular polymeric substances acting as protective shields that keep the ACC stable in water-rich environments at room temperature. This is like having tiny factories that can manufacture the raw materials for bone repair right where they’re needed most. When bacterial cells die, the protective layer breaks down, allowing the ACC to crystallize into calcite, with bacteria capable of producing up to 20% ACC compared to calcite depending on colony age. Think of it as nature’s own 3D printer, but instead of plastic, it’s printing the building blocks of bone. Microscopic imaging reveals that bacteria aggregate and trap free calcium ions, providing nucleation sites for crystal formation. These bacterial communities work together like a construction crew, each playing a specific role in the mineral-building process. The precision is astounding—these organisms can control exactly where, when, and how much mineral gets deposited.
The French Connection to Bone Healing

French researchers have been at the forefront of discovering cave bacteria’s potential for medical applications, building on the country’s rich tradition of speleotherapy—using cave environments for healing. Speleotherapy has been used historically in many caves, proving more efficient than mountain sanatorium treatments for respiratory diseases. But the real breakthrough came when scientists realized that the same bacterial processes creating cave formations could be harnessed for bone repair. Studies of dripping water samples from caves revealed diverse heterotrophic bacterial communities with different colony morphologies capable of calcium carbonate precipitation. French cave systems, with their unique geological and climatic conditions, have proven to be goldmines for bacteria with exceptional biomineralization capabilities. The genera Stenotrophomonas, Bacillus, and Rhodococcus have been commonly identified in cave environments, each bringing its own special talents to the bone-healing party. These aren’t your ordinary bacteria—they’re specialized mineral architects that have spent eons perfecting their craft.
Bacterial Bone Builders: The Science Behind the Magic

The microbially catalyzed biomineralization process involves three key stages: bacterial adhesion to biomaterials, production of carbonate ions assisted by bacteria, and nucleation and growth of calcium carbonate nanocrystals on bioceramic surfaces. It’s like watching a choreographed dance at the molecular level, where each bacterial cell knows exactly when and where to perform its part. The resulting microbially catalyzed biominerals exhibit uniform micro and nanostructures on both 2D and 3D bioceramics, presenting excellent bone-forming bioactivity both in laboratory and living organism studies. Picture millions of microscopic construction workers, each equipped with the perfect tools to build exactly what your broken bone needs. Bacterial cell surface molecules, including cell walls and extracellular polymeric substances, play crucial roles in binding and concentrating calcium ions for carbonate precipitation. These bacteria essentially turn themselves into living scaffolds, creating the perfect environment for new bone tissue to grow. The precision is mind-boggling—they can control crystal size, shape, and even the type of calcium carbonate produced.
From Cave Floor to Operating Room
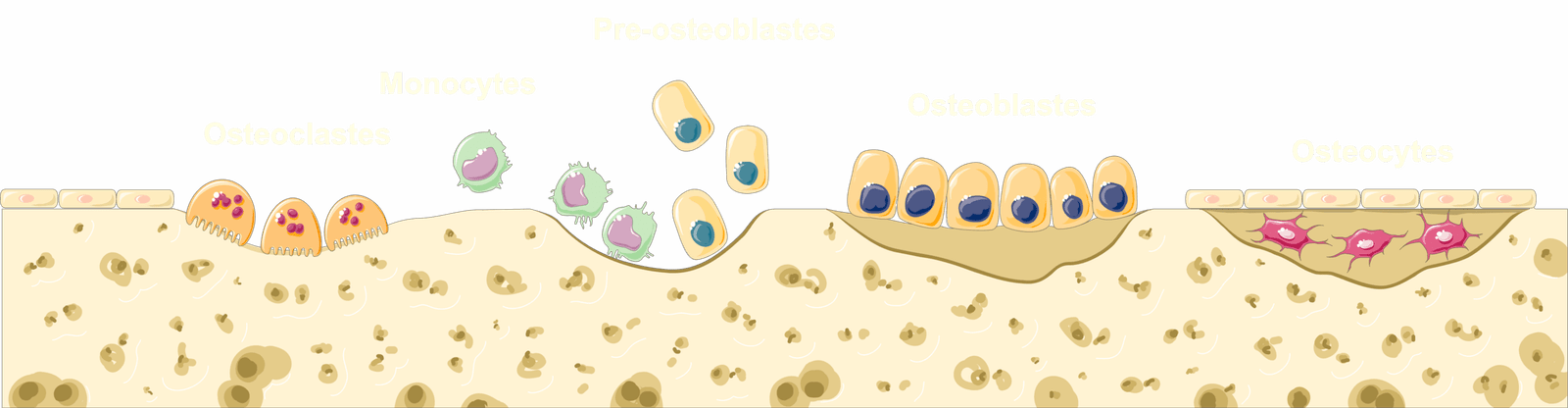
Research has shown that Bacillus subtilis strains isolated from Brazilian Amazon caves demonstrate significant calcium carbonate precipitation capacity, with crystals displaying typical calcite morphology. But the real excitement comes from their potential medical applications. Preliminary tests show that cave-isolated bacterial strains in mortar systems demonstrate crack self-healing abilities and increased compressive strength after 63 days, without requiring additional urea or calcium sources. Now imagine applying this same self-healing principle to fractured bones. Researchers have developed scaffold-like materials infused with copper nanoparticles that kill harmful bacteria while incorporating genetic molecules to stimulate new bone formation. Laboratory tests showed these scaffolds could stimulate bone regrowth in just two weeks while stopping 80% of potentially harmful bacteria from attaching to the treatment site. It’s like having a smart bandage that not only helps your bone heal faster but also fights off infection at the same time.
The Calcium Carbonate Connection
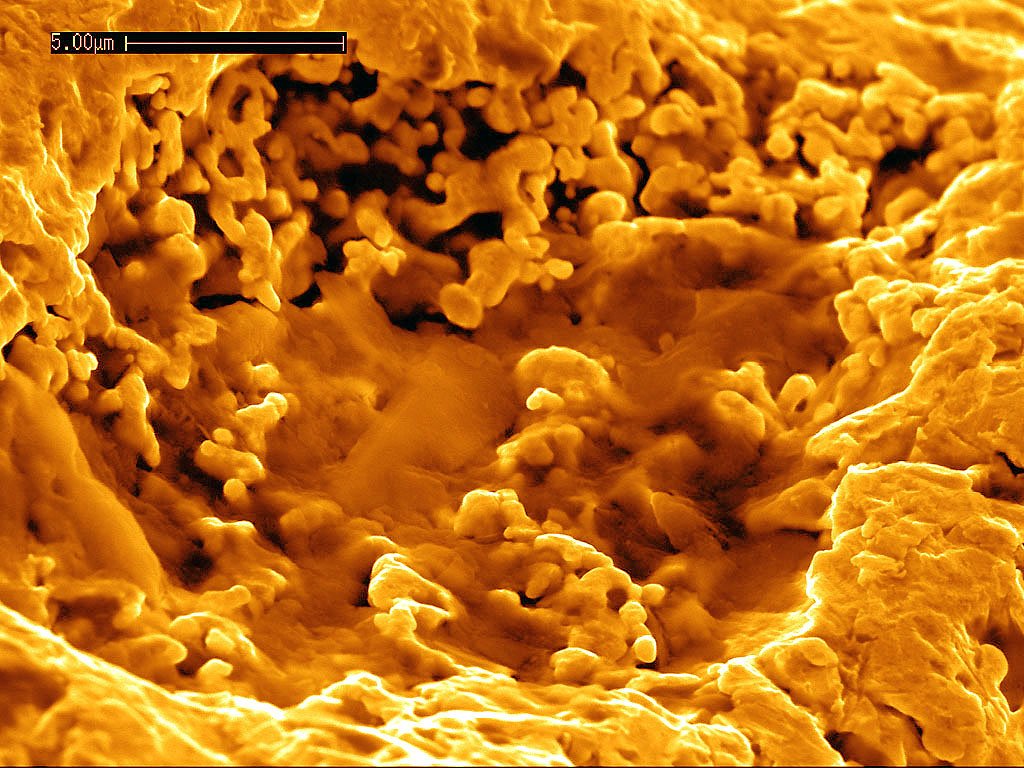
Cave bacteria strains have been identified as capable of precipitating calcium carbonate, with species like Bacillus toyonensis, Paracoccus limosus, and Brevundimonas diminuta showing particularly strong mineralization abilities. These aren’t just random bacteria—they’re nature’s selected specialists in bone-building chemistry. The precipitated calcium carbonate shows dominance of calcite, aragonite, and nano-sized vaterite, with these bacterial communities actively contributing to formation by enhancing the pH of their microenvironment. Think of them as tiny pH adjusters, creating the perfect chemical conditions for bone minerals to form naturally. Even in extreme cold conditions, bacteria can actively participate in calcium carbonate precipitation, with cell surface molecules and extracellular polymer substances influencing crystal nucleation and formation. This means these bacterial bone-healers could potentially work in various body conditions and temperatures. The production of specific bacterial outer structures and their chemical nature proves crucial for the crystallization process, influencing both the mineralogy and morphology of calcium carbonate crystals.
Revolutionary Treatment for Bone Infections

Osteomyelitis, a devastating bone infection, has high recurrence rates and impaired bone restoration, making it one of the most challenging medical conditions to treat. Traditional treatments often fail because bacteria form protective biofilms that shield them from antibiotics and immune responses. Despite the best medical care, osteomyelitis treatment failure rates range from 10% to 40%, with Staphylococcus aureus accounting for over 70% of these infections. But cave bacteria might offer a game-changing solution. The nutrient-poor cave environments stimulate competition among microbes, causing them to develop survival strategies including the secretion of metabolites with antibiotic properties. Cave bacteria have spent trillions of years acquiring chemical weapons to inhibit other bacteria, making cave ecosystems favorite environments for discovering microbes with active compounds. These bacterial warriors could potentially fight bone infections while simultaneously helping to rebuild the damaged tissue.
Nature’s 3D Printing Technology

Biomineralization represents the process by which organisms form mineralized tissues with hierarchical structures and excellent properties, providing inspiration for designing materials to repair hard tissues. Cave bacteria have mastered this process over millions of years of evolution. The formation processes of minerals can be partly replicated using bioinspired artificial materials to mimic biomolecule functions or stabilize intermediate mineral phases involved in biomineralization. It’s like reverse-engineering nature’s most sophisticated manufacturing process. Biologically controlled mineralization occurs when crystal morphology, growth, composition, and location are completely controlled by cellular processes, with collagen mineralization providing crucial compressive strength for bones, cartilage, and teeth. Bacterially induced calcium carbonate precipitation can be used to produce self-healing materials, with bacteria triggering mineral precipitation that can reseal cracks and provide structural support. Imagine having bacteria that automatically detect and repair microscopic fractures in your bones before they become serious problems.
The Perfect Storm for Bone Regeneration

Researchers have developed composite mineral matrix hydrogels containing amorphous calcium phosphates, inspired by natural biomineralization processes, creating dynamic networks through calcium ion coordination. These materials mimic what cave bacteria do naturally. In natural biominerals like teeth and bones, non-collagenous proteins and proteoglycans act as binders and stabilizers of amorphous calcium phosphate precursors, forming the basis for unique mechanical properties. Cave bacteria have essentially evolved to perform the same function as these expensive biological molecules, but they can be cultivated and harvested much more affordably. The biomineralization process in organisms creates mineralized tissues with hierarchical structures and excellent properties, with underlying mechanisms providing inspiration for hard tissue repair materials. The formation processes can be replicated using bioinspired materials to mimic biomolecule functions or stabilize intermediate mineral phases involved in biomineralization. French cave bacteria represent nature’s own research and development team, having already solved the engineering challenges we’re just beginning to understand.
Overcoming Traditional Treatment Limitations
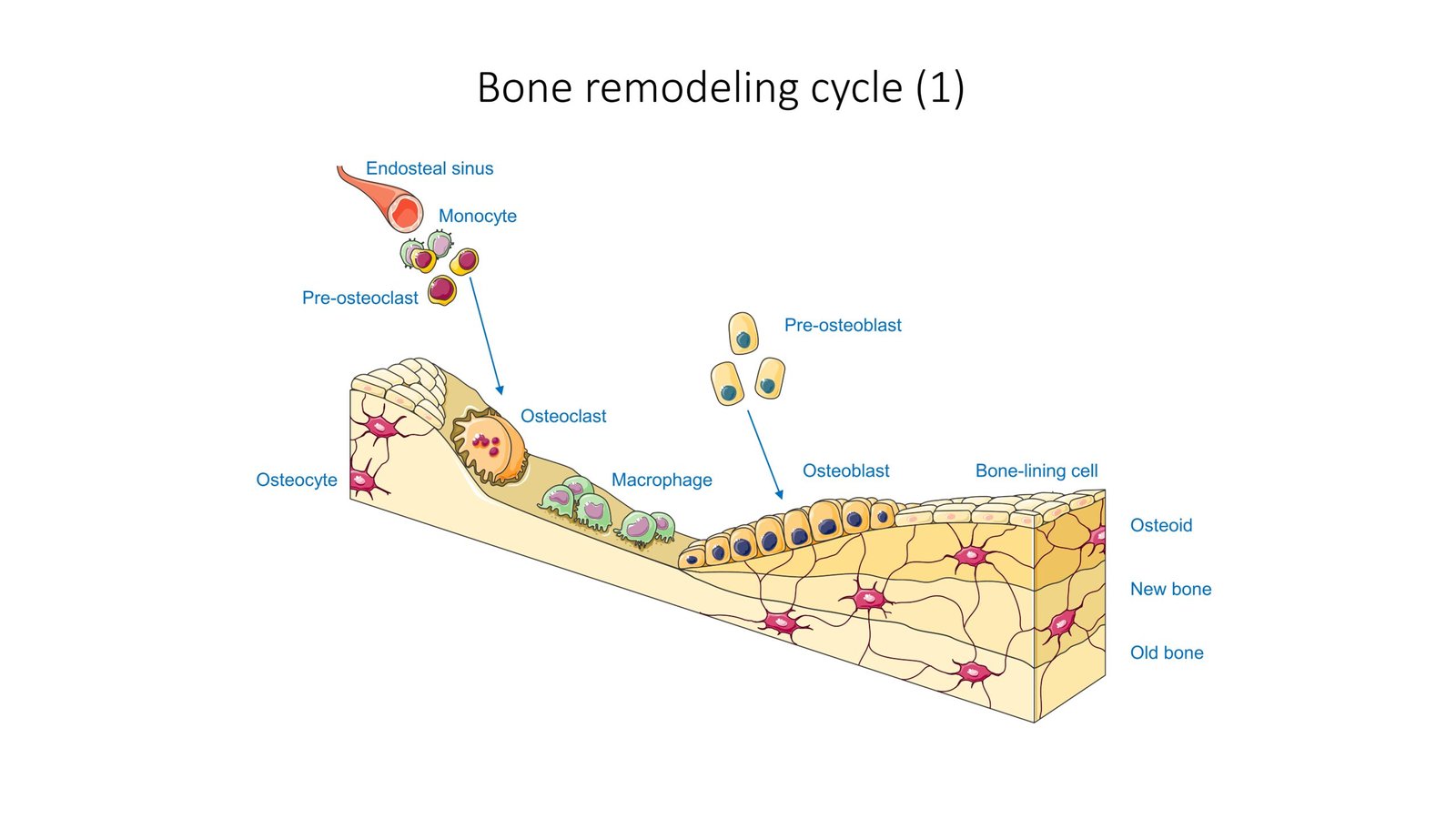
While autogenous bone remains the gold standard for bone defect repair due to excellent bone conduction and biocompatibility, major problems include insufficient tissue, donor-site injury, nerve and vascular damage, chronic pain, and surgical risks. Cave bacteria could potentially eliminate many of these complications. To solve clinical problems of existing scaffolds for hard tissue regeneration, researchers are developing drug-free inorganic biomaterial platforms that respond to bacterial infection and osteomyelitis. Under mild inflammatory conditions, pro-inflammatory cytokines or bacteria-derived antigens can promote osteoblast differentiation, but sustained inflammation from high-dose bacterial stimuli leads to predominantly bone destruction. The key is finding the right balance, and cave bacteria might naturally provide that perfect equilibrium. Low-dose bacterial stimuli can promote bone formation, while high-dose stimuli lead to bone destruction, highlighting the importance of controlled bacterial application. These cave organisms have already mastered the art of controlled mineral deposition over millions of years.
Advanced Imaging Reveals Bacterial Architecture
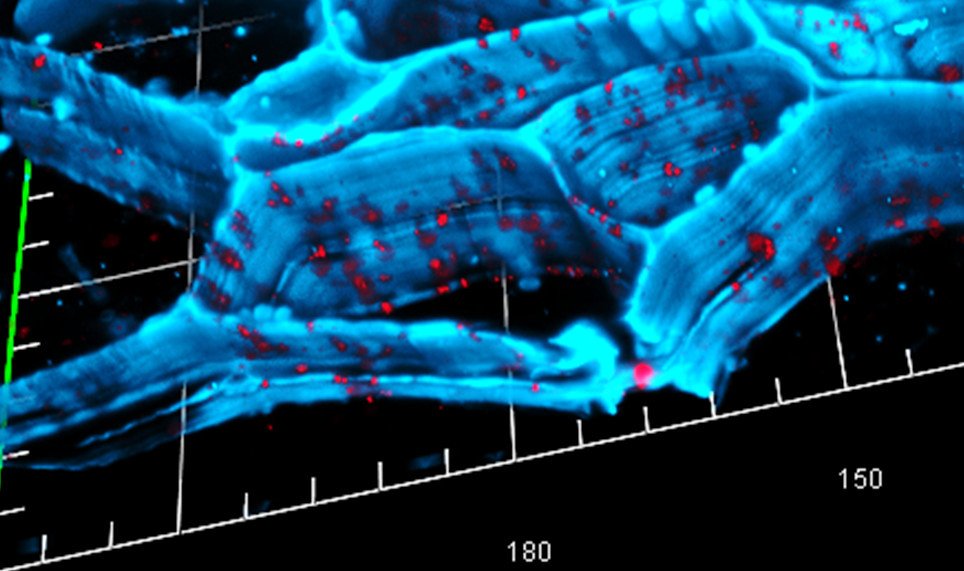
Confocal laser scanning microscopy of cave bacteria reveals how they aggregate and trap free calcium ions, with the ions appearing green while the bacterial structures show up in white. It’s like watching tiny molecular factories in real-time action. Energy-dispersive X-ray mapping shows that different bacterial strains produce calcium carbonate minerals with distinct shapes—round when precipitated by Arthrobacter sulfonivorans but idiomorphous when formed by Rhodococcus globerulus. This means doctors could potentially select specific bacterial strains based on the exact type of bone repair needed. The cell walls of different bacterial species have varying surface properties, with some being more hydrophilic and others more hydrophobic, directly influencing the type and shape of calcium carbonate minerals produced. The amount of peptidoglycan and degree of amidation of cell surface components influence cell surface charge, causing observed differences in calcium carbonate mineral morphology. It’s precision medicine at the bacterial level, where the microscopic architecture of each bacterial strain determines its bone-building capabilities.
The Antibiotic Factory Underground

Cave ecosystems have become favored environments for discovering novel bioactive compounds, with bacteria having spent trillions of years acquiring chemical weapons to inhibit other bacterial growth. These aren’t just bone builders—they’re also potential infection fighters. Pre-historic people used moon-milk, white cave exudates, to treat many diseases, which shows potential against multiple bacteria and fungi. Our ancestors might have been onto something profound without even knowing it. The first antifungal drug was discovered in a bacterial strain, and bacterial populations continue to be the best source of antifungal drugs, with ecological interactions between fungi and bacteria helping us understand bioactive compound evolution. Cave environments create stress for inhabitant microorganisms at the genetic level, making them reservoirs of novel microbial species and bioactive compounds, though only a few metabolites have had their chemical structures identified. French caves represent untapped pharmaceutical goldmines, where every bacterial strain could potentially yield new antibiotics or bone-healing compounds.
Clinical Translation: From Laboratory to Patient
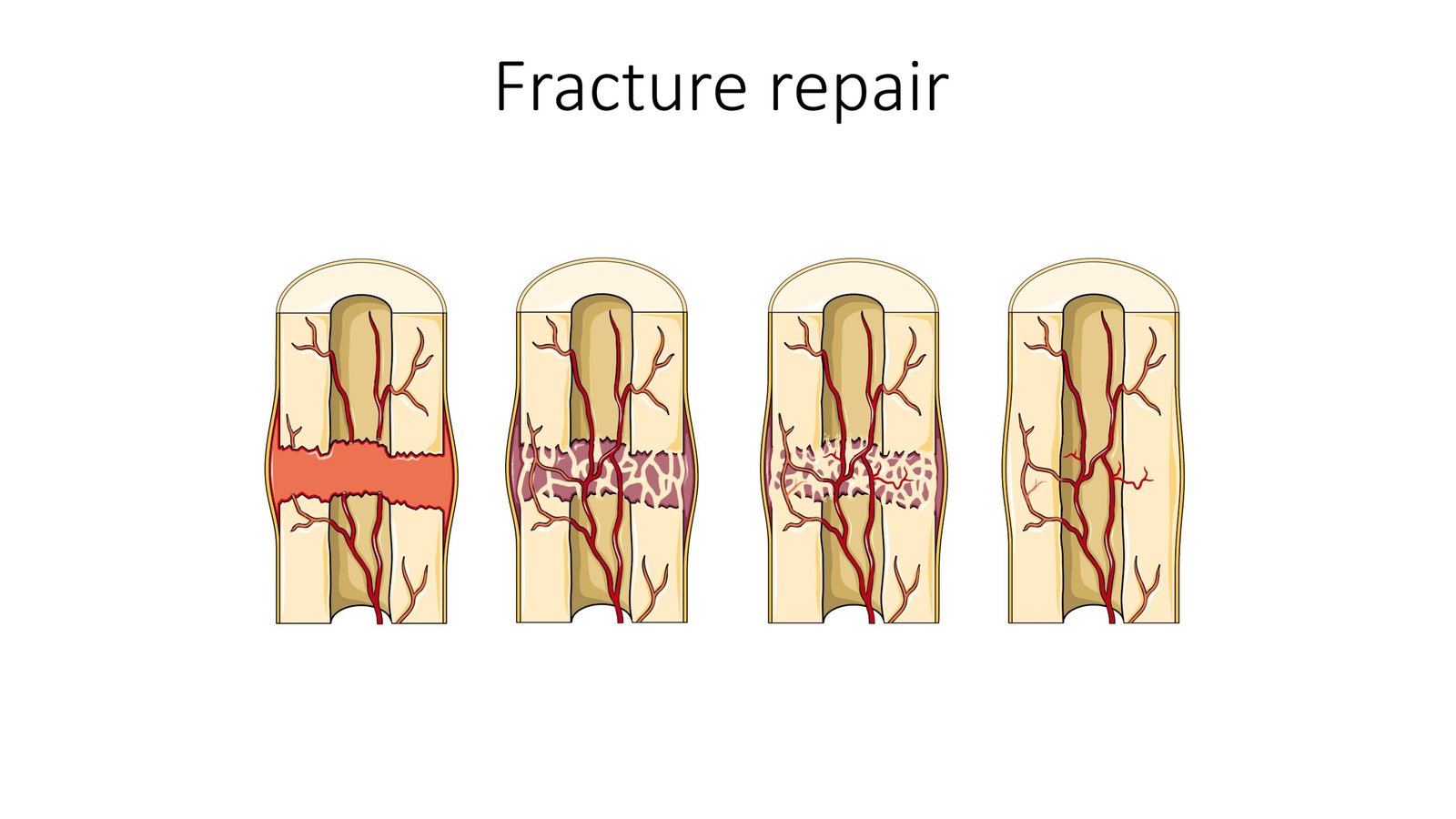
The fusion of bioactive material systems into collagen-apatite-based scaffolds, combined with oxygen and nutrient delivery to attract body cells, shows great potential for clinical translation. This represents the bridge between cave bacteria discoveries and actual patient treatment. Research focuses on biocompatibility, osteoconductivity, and biomineralization in developing bio-scaffolds for hard tissue regeneration, with successful biomineralization of synthesized materials achieved in the past decade representing significant progress toward authentic biomimetic bone-like structures. Continuous supply of bone-building proteins promotes osteogenic differentiation and biomineralization, with the immune microenvironment being crucial for bone tissue regeneration. Macrophages can polarize into pro-inflammatory M1 phenotypes that stimulate inflammation, or pro-healing M2 phenotypes that aid in stem cell bone formation and tissue remodeling. Cave bacteria might naturally trigger the beneficial M2 response while suppressing harmful M1 inflammation, creating the perfect healing environment.
Environmental Factors and Bacterial Performance
Enhanced calcium carbonate precipitation by cave bacteria occurs at 25°C and pH 5, with precipitation confirmed through advanced imaging and spectroscopy techniques. Understanding these optimal conditions is crucial for therapeutic applications. Psychrophilic bacteria adapt to low temperatures by modifying their cell wall and extracellular polymer compositions, with lipid and choline contents varying with incubation temperature. This adaptability means cave bacteria could potentially function across different body conditions and patient needs. Major metabolic processes involved in microbially induced carbonate precipitation include photosynthesis, ammonification, ureolysis, and various reduction processes as well as methane oxidation. Cave diagenesis alters crystallinity and ordering of hydroxyapatite, with recrystallization strongly influenced by calcium carbonate-rich dripping water leading to higher crystallinity crystal formation. The cave environment itself acts as a natural laboratory for optimizing these bacterial processes.