Some of the biggest scientific breakthroughs didn’t come from grand plans or perfectly executed experiments. They came from a quiet moment of noticing something odd, from a mistake no one meant to make, or from someone simply paying attention when others would’ve walked away. That’s the strange, thrilling power of observation: it can turn an everyday accident into a discovery that rewrites our understanding of the world.
When you dig into the stories behind these breakthroughs, a pattern appears. It’s not just luck. It’s curiosity meeting chance. It’s a stubborn refusal to ignore the weird, the inconvenient, or the “this shouldn’t be happening” moment. Let’s walk through nine accidental discoveries that prove how a single observation, caught by the right pair of eyes, can change science forever.
1. Penicillin: The Mold That Saved Millions

Imagine walking into your lab, expecting neat rows of bacterial cultures, and instead finding one plate ruined by fuzzy mold. Most people would toss it in the trash. Alexander Fleming didn’t. In 1928, he noticed that the bacteria growing near the invading mold were mysteriously dying while the rest of the plate flourished. That tiny clear ring around the mold didn’t look important at first glance, but it quietly defied everything he expected to see.
Fleming’s decision to investigate that contamination instead of discarding it led to the discovery of penicillin, the first true antibiotic. It took years, and the efforts of other scientists, to turn that observation into a drug that could be mass-produced, but the spark was simple: he observed carefully, and he wondered why. Today, antibiotics based on that first chance finding have saved countless lives, especially from infections that used to be a near-certain death sentence. One forgotten Petri dish turned out to be more powerful than any carefully planned experiment he ran that year.
2. X-Rays: A Glowing Screen and Invisible Light

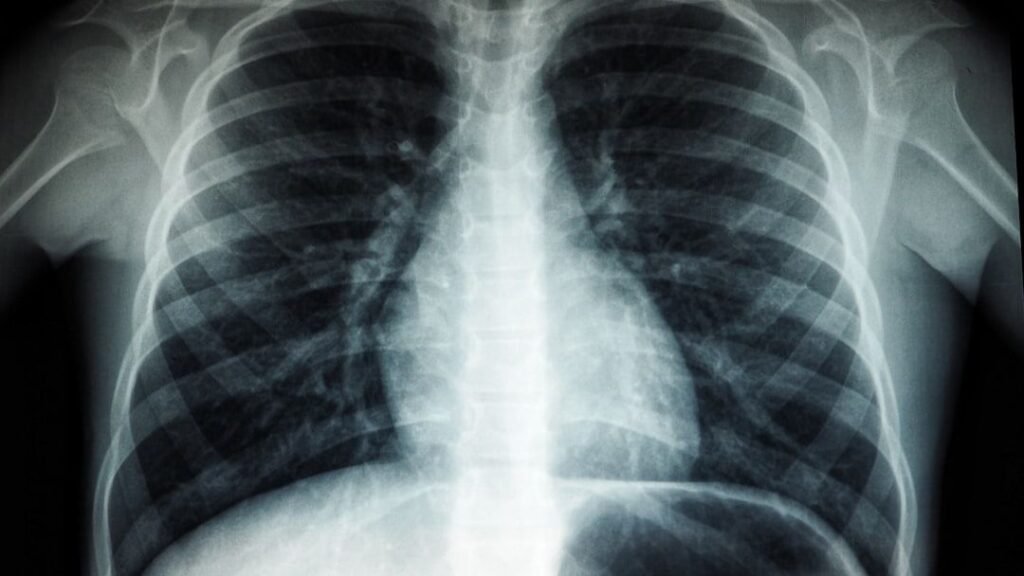
In 1895, Wilhelm Röntgen was studying cathode rays in a darkened lab when he saw something strange: a fluorescent screen across the room started glowing, even though it shouldn’t have. The rays he was working with weren’t supposed to travel through metal or make anything light up from a distance, yet his screen clearly reacted. Most people might have chalked it up to a glitch in the equipment or ignored it as some odd side effect.
Röntgen, however, did the opposite and leaned into the weirdness. He began placing objects between the tube and the screen and noticed their shadows, including the bones inside his own hand, appearing as ghostly shapes. That was the birth of X-rays, a completely new kind of electromagnetic radiation that could pass through soft tissue but not bone or metal. Medical imaging, airport security, and modern diagnostics all trace back to that one eerie glow in a dark room, noticed by someone who refused to dismiss it.
3. Microwave Ovens: A Melted Candy Bar in a Scientist’s Pocket

During World War II and the years after, engineers were working intensely with radar equipment and magnetrons, the devices that generate high-powered microwaves. In the 1940s, Percy Spencer, an engineer working near an active radar set, noticed something odd: the chocolate bar in his pocket had melted into a mess. It wasn’t a hot day, and he wasn’t standing next to an oven, yet his snack had turned to goo while he worked.
Instead of just being annoyed about losing his candy, Spencer got curious and started experimenting. He placed popcorn kernels near the radar equipment and watched them pop. Then he tried an egg, which famously exploded. These odd results led him to realize that microwaves could be used to heat food quickly and efficiently. The first commercial microwave ovens were bulky and expensive, but they opened the door to a new kind of cooking that many homes now take for granted, all born from a simple observation: a melted candy bar in the wrong place at the right time.
4. Cosmic Microwave Background: Static That Mapped the Early Universe

In the 1960s, two engineers, Arno Penzias and Robert Wilson, were trying to fine-tune a sensitive radio antenna in New Jersey. No matter what they did – cleaning away nesting pigeons, adjusting equipment, recalibrating receivers – they kept picking up a faint, persistent noise. It was like a stubborn static that refused to disappear, regardless of where they pointed the antenna in the sky. To them, at first, it felt like an annoying technical problem rather than a profound clue.
But that faint hiss turned out to be the cosmic microwave background, the cooled echo of the Big Bang itself. It was the afterglow of the early universe, stretched and chilled as space expanded over billions of years. Their accidental observation became one of the strongest pieces of evidence for the Big Bang theory and transformed cosmology into a precision science. What started as an irritating, unexplained buzz ended up as a map of the infant universe, all because they refused to shrug off the noise.
5. Radioactivity: Darkened Plates and an Unexpected Force

In the late nineteenth century, Henri Becquerel was investigating materials that glowed after exposure to sunlight. He thought uranium salts might produce similar effects when illuminated, so he wrapped them with photographic plates and left them in the sun. Cloudy weather interrupted his plans, and he stored the wrapped plates in a drawer, assuming the experiment had been a dud. When he later developed the plates, he found strong images as if they had been exposed to bright light.
That result made no sense under the original idea: the salts hadn’t seen sunlight, yet they had still affected the plates. Becquerel realized the uranium was emitting a new kind of penetrating energy all on its own. This accidental discovery of radioactivity was later built upon by scientists like Marie and Pierre Curie, who explored radioactive elements in depth. Radioactivity would go on to reshape physics, medicine, and energy production, but it all started with a failed weather-dependent experiment and a sharp eye on a supposedly ruined batch of photographic plates.
6. Vulcanized Rubber: A Spill on a Hot Stove

In the early 1800s, Charles Goodyear became obsessed with improving natural rubber, which was sticky in the heat and brittle in the cold. He tried additive after additive, often ending up with foul-smelling, useless messes. One day, as the story goes from historical accounts, a mixture of rubber and sulfur accidentally came into contact with a hot stove. Instead of melting into a puddle, the material charred slightly but remained stretchy and resilient.
Goodyear’s observation of this odd behavior led him to realize that heat and sulfur together could transform rubber into something far more durable. He refined the process into what we now call vulcanization, a treatment that makes rubber stable across a wide range of temperatures. That accident on a hot surface became the foundation for everything from car tires to shoe soles and countless industrial products. Without that single, messy moment of burning rubber and a curious mind, everyday life in the modern world would literally run very differently.
7. Safety Glass: A Shattered Flask That Didn’t Shatter

In the early 1900s, French chemist Édouard Bénédictus reportedly dropped a glass flask coated on the inside with a thin layer of plastic cellulose solution. Instead of exploding into sharp, dangerous shards, the glass cracked but held together, clinging to the transparent film. That behavior was surprising in an era when broken glass was almost always a hazard waiting to slice anyone nearby. It was the kind of accident most people would clean up and forget in seconds.
Bénédictus, however, saw potential in that odd crack pattern. By intentionally laminating glass with plastic, he created a material that stayed largely intact when broken, greatly reducing the risk of injury. This principle became the basis for safety glass, now standard in car windshields and many building windows. The key shift came from looking at a dropped flask not just as broken equipment, but as a hint that glass could fail more safely, if only we designed it that way.
8. Teflon: A Gas That Disappeared into a Slippery Solid

In 1938, chemist Roy Plunkett was working with gases related to refrigerants, storing them in pressurized cylinders. When he opened one cylinder that should have contained gas, nothing came out. It seemed empty, but the weight was unchanged. Curious, he cut the cylinder open and found a strange, waxy white solid coating the inside rather than the expected gas. For many people, that would have been a frustrating equipment failure and little more.
Instead, Plunkett and his team tested the material and discovered it had remarkable properties: it was extremely slippery, chemically inert, and could withstand high temperatures. That substance became polytetrafluoroethylene, better known as Teflon. Over time it found uses in nonstick cookware, aerospace components, and countless industrial applications. The entire line of products grew out of noticing that a gas had mysteriously turned into a solid and deciding to investigate what, exactly, had happened inside that steel cylinder.
9. Saccharin and Artificial Sweeteners: A Forgotten Handwashing Step

In the late nineteenth century, chemist Constantin Fahlberg was working with coal tar derivatives in a lab, studying compounds related to food preservatives. After a day of work, he went home and ate dinner, only to notice that his bread and other foods tasted unusually sweet. Eventually, he traced the sweetness back to his own hands, realizing he had forgotten to wash them properly after handling a particular compound. That oversight might seem careless, but it led to a stunning realization.
Fahlberg identified the responsible substance and named it saccharin, one of the first artificial sweeteners. Over time, artificial sweeteners have sparked debates around health, diet culture, and food regulation, but they have also offered options for people needing to limit sugar intake, such as those with diabetes. This entire field emerged because someone paid attention to a strange taste at the dinner table and connected it to what happened at the lab bench hours earlier. It’s a reminder that scientific observation doesn’t switch off when you leave work; sometimes, the crucial clue shows up on your plate.
The Quiet Power of Paying Attention

Across all these stories, one common thread stands out: the discoveries weren’t planned, but the people behind them were prepared to notice. They didn’t just follow their hypotheses; they watched closely when things went wrong, when instruments misbehaved, or when everyday experiences felt a little off. Their accidental finds changed medicine, physics, chemistry, engineering, and even how we cook our food and drive our cars.
In a way, these breakthroughs are both humbling and empowering. They show that the universe constantly offers hints, glitches, and surprises, and that the real magic happens when someone chooses to look twice instead of looking away. You don’t need a perfect plan to uncover something important – you need curiosity, patience, and the willingness to treat a mistake as a message instead of a failure. The next time something doesn’t go as expected, what might you see if you really stop and observe?