Picture a hospital in 2050 where a diagnosis of a genetic disease prompts a single, precise edit – then a life resumes its ordinary rhythm. That future feels tantalizingly close after the first wave of CRISPR-based therapies proved one-time treatments can work in people. Yet every step forward reveals new knots: complex genetics, hard-to-reach organs, and the realities of cost and access. The question isn’t whether CRISPR will change medicine – it already has – but whether it can reach the full sweep of genetic disease in time. The answer depends on how science, policy, and equity move in the next two decades.
The Hidden Clues

Behind the headline breakthroughs lies a sobering map of illness: thousands of rare, single-gene disorders scattered across every organ system. Many strike in childhood, often with no approved therapy, and families pin hopes on experimental trials that may never come their way. Genome sequencing now flags causal variants faster than ever, turning a diagnostic odyssey into a targeted plan.
That shift matters because CRISPR works best when the enemy is clear and singular – a well-defined mutation, a cell type we can reach, and a path to measure success. Metabolic diseases in the liver, blood disorders in bone marrow, and some eye diseases fit that playbook. But even in these, timing, delivery, and durable benefit determine whether a fix is practical or just possible.
From Scissors to Swiss‑Army Genes
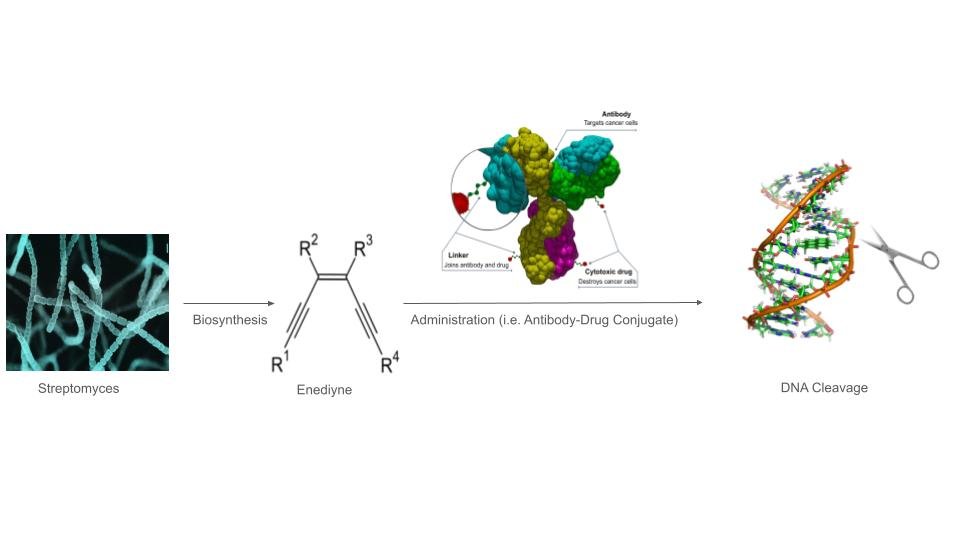
CRISPR began as a cut-and-paste idea, using enzymes like Cas9 to slice DNA and let cells repair the break. The toolkit has since exploded: base editors swap single letters without cutting both strands, and prime editors write short patches of new code with surgeon-like finesse. Researchers are also steering editors to switch genes off, flip them on, or tweak RNA instead of DNA.
Therapies take two main routes. Ex vivo approaches edit cells outside the body – like blood stem cells – then reinfuse them, a strategy already changing care for inherited blood diseases. In vivo approaches send editors directly to tissues, promising single-shot fixes but demanding delivery systems that are safe, precise, and potent at clinical scale.
Why It Matters
For patients, genetic disease is rarely a single crisis; it’s a slow, relentless erosion of choices. Traditional options – bone marrow transplants, enzyme infusions, or chronic drugs – can work but ask for years of risk and maintenance. A one-time edit that silences the root cause can turn a lifetime of care into a recovery arc.
I’ve covered CRISPR long enough to see something subtle: families start planning futures again, not just schedules around hospitals. That psychological pivot is enormous – school, work, travel, all reorganized around possibility rather than limitation. If CRISPR can reliably deliver durable benefit, it reshapes not only medicine’s ledger but also people’s time, which might be the most valuable outcome of all.
Delivery: The Last Mile into the Right Cell
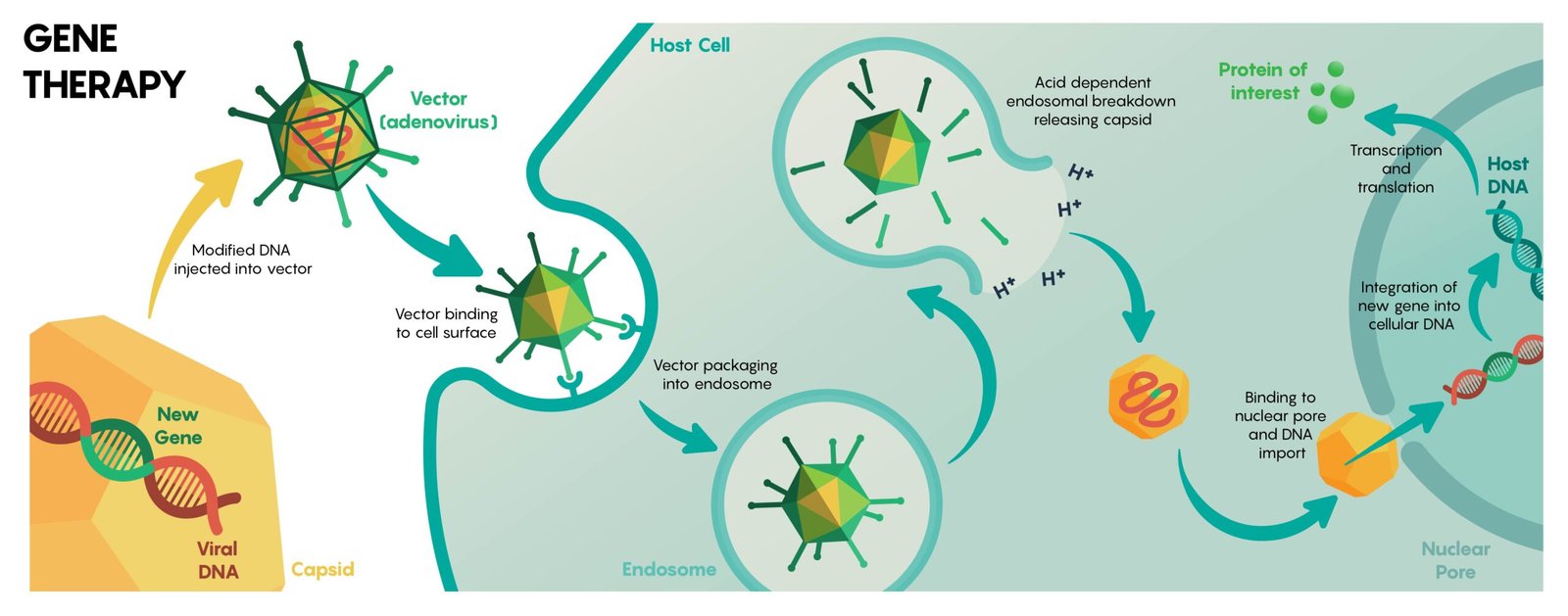
Every genetic editor needs a ride, and that ride is the constraint that decides winners. Lipid nanoparticles ferry RNA-guided editors into the liver efficiently, which is why many early successes target hepatic genes. Viral vectors reach other tissues better but bring size limits and immune baggage that can complicate repeat dosing.
The hardest neighborhoods remain the brain, heart, and lungs – densely protected, constantly moving, or both. Engineers are evolving smarter carriers with tissue homing tags, smaller enzymes that fit tight viral capsids, and nonviral particles tuned for specific cell types. Mastering this last mile is the difference between treating dozens of diseases and treating thousands.
Safety, Standards, and Trust

Cutting DNA is powerful, and power invites scrutiny. Off-target edits, large genomic rearrangements, and unexpected immune reactions are risks that regulators and researchers now monitor with deeper sequencing and longer follow-up. The field is moving toward shared safety playbooks: standardized assays, transparent registries, and long-term surveillance to catch late effects.
Safety is also about avoiding the wrong promise. Some mutations look fixable on paper but sit in cells that turn over too slowly, or in tissues that can’t be reached without undue risk. Trust grows when trials choose indications where biology, delivery, and clinical endpoints align – and when setbacks are reported with the same clarity as successes.
Global Perspectives
Genetic disease doesn’t respect borders, and neither should solutions. Regions with the highest burden of inherited conditions often have the least access to advanced therapies, a mismatch that technology alone cannot solve. Building local manufacturing, training clinical teams, and modernizing regulations will matter as much as the next clever enzyme.
Policy lines also vary: many countries bar heritable (germline) edits while allowing somatic therapies that treat only the patient. That balance reflects a broad consensus – fix illness without rewriting inheritance – but it must be revisited as tools improve. Without global coordination, access gaps widen, and black‑market shortcuts tempt those left waiting.
The Hard Stops We Can’t Wish Away

Not every genetic disease is a single typo; many are woven from thousands of variants interacting with environment and chance. Editing risk across such polygenic webs may prove scientifically unwieldy and ethically fraught, especially when small gains require many simultaneous changes. Even some single‑gene disorders damage tissues before birth, narrowing the window where editing can help.
There are practical ceilings too: manufacturing capacity, clinical staff, and the cost of individualized, autologous procedures. List prices for first-generation gene therapies have landed in the million‑dollar range, and while competition and scale may lower costs, the curve won’t bend by goodwill alone. Financing models, public–private partnerships, and simpler, off‑the‑shelf products must evolve in parallel with the science.
The Future Landscape
The next decade is about precision without collateral: prime editing in vivo, editors that slide seamlessly into and out of cells, and enzymes engineered for narrower footprints and tighter specificity. Programmable integration systems aim to insert larger DNA segments safely, opening doors for diseases that need gene repair rather than gene silencing. On the analytics side, single‑cell multi‑omics will show exactly which cells were edited, how they behave months later, and where micro‑failures hide.
By 2050, a plausible outcome is bold yet bounded: most treatable single‑gene diseases have at least one viable therapy; a subset will be addressed by one‑and‑done edits; complex, polygenic disorders will rely on prevention and non‑editing tools. The revolution is real, but it likely stops short of “every” genetic disease – biology prefers nuance to absolutes. That’s not a failure; it’s a blueprint.
What You Can Do Now

Follow trials that matter to your community and learn the basics of how each edit works, including what success actually means for daily life. Support newborn screening and genetic counseling programs that connect families to appropriate trials faster and more fairly. Patient registries, often overlooked, are the quiet engines that make studies possible and representative.
Advocate for policies that expand access: insurance coverage for genomic testing, funding for local manufacturing, and ethical guardrails that protect patients without freezing progress. If you’re in a position to help, back nonprofits that train clinicians and build lab capacity where disease burden is highest. Change here is cumulative, and every early brick makes the future sturdier – what part will you lay today?

Suhail Ahmed is a passionate digital professional and nature enthusiast with over 8 years of experience in content strategy, SEO, web development, and digital operations. Alongside his freelance journey, Suhail actively contributes to nature and wildlife platforms like Discover Wildlife, where he channels his curiosity for the planet into engaging, educational storytelling.
With a strong background in managing digital ecosystems — from ecommerce stores and WordPress websites to social media and automation — Suhail merges technical precision with creative insight. His content reflects a rare balance: SEO-friendly yet deeply human, data-informed yet emotionally resonant.
Driven by a love for discovery and storytelling, Suhail believes in using digital platforms to amplify causes that matter — especially those protecting Earth’s biodiversity and inspiring sustainable living. Whether he’s managing online projects or crafting wildlife content, his goal remains the same: to inform, inspire, and leave a positive digital footprint.




