Polycystic ovary syndrome can feel like a riddle passed down the family tree: a grandmother’s irregular cycles, a mother’s fertility struggles, a daughter’s metabolic worries. For years, the story leaned heavily on genetics and lifestyle, yet neither quite explained why the condition echoes so persistently across time. Now, a wave of research is pointing at an unexpected narrator – the egg cell itself – and the chemical “tags” it carries. These epigenetic marks don’t rewrite DNA, but they can nudge genes louder or quieter, shaping development from the very first moments. I still remember sitting in a clinic waiting room, watching three generations compare notes, and wondering if biology might be keeping score before birth.
The Hidden Clues

What if the story of PCOS begins before you were even born, etched as faint instructions inside an egg cell? That’s the provocative idea gaining traction as scientists map epigenetic marks – chemical signposts that guide which genes switch on in early embryos. Unlike mutations, these tags can be added or erased by environment, hormones, or metabolism, leaving a residue of experience on biology.
In PCOS, researchers are finding that some of these marks differ in oocytes and very early embryos from affected families. It suggests a mechanism for why PCOS can appear in daughters and even granddaughters, beyond standard inheritance. The egg, in other words, may carry a memory of maternal physiology that subtly programs the next generation.
From Mice to Mothers: Tracing the Signal

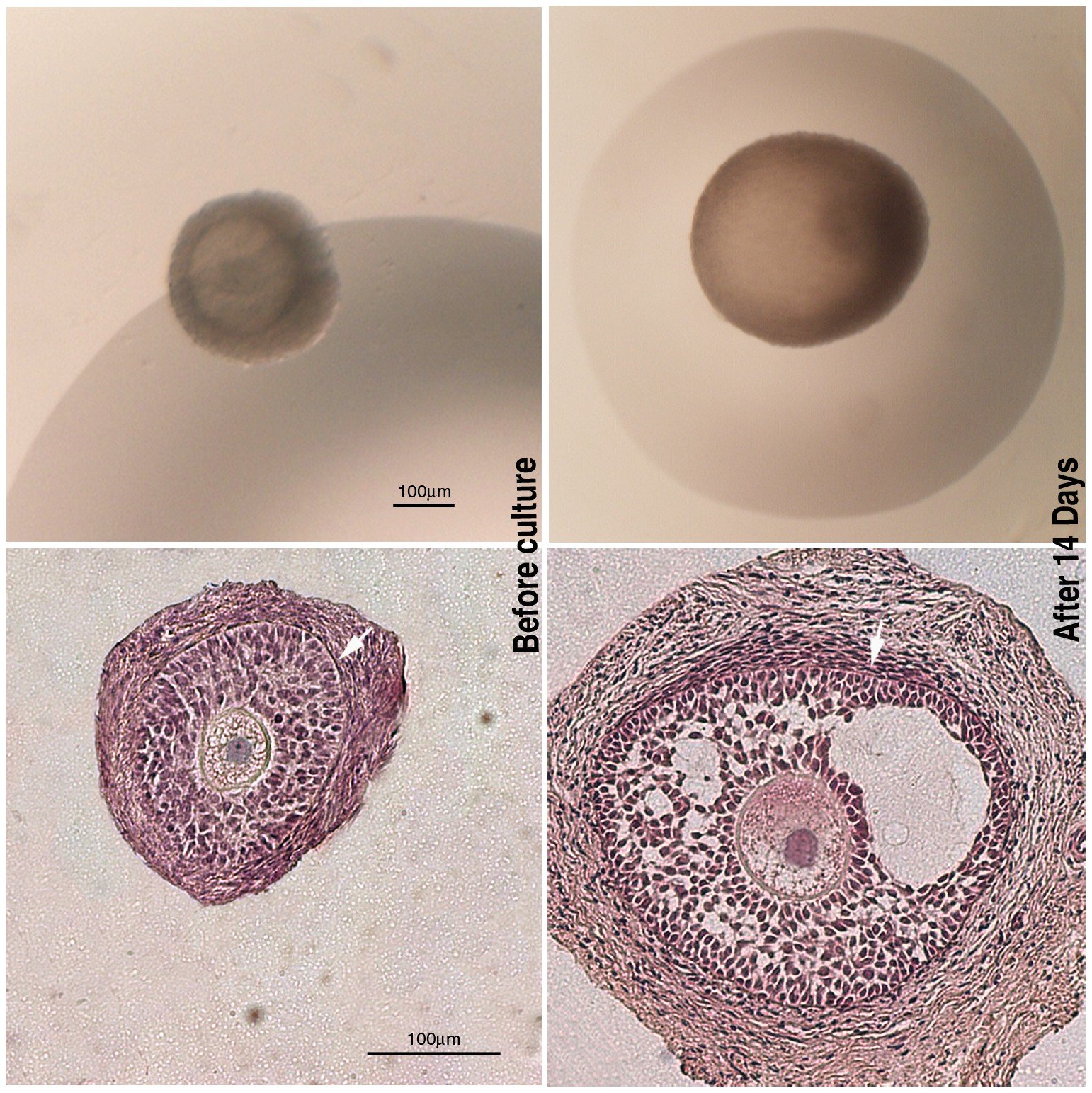
Animal models have been crucial for testing this idea because they let scientists separate the egg’s contribution from the uterus or postnatal environment. In carefully controlled experiments, eggs taken from females with PCOS-like traits can transmit metabolic and reproductive problems to offspring – even when embryos develop in healthy surrogate mothers. That points squarely at the oocyte’s epigenetic script as a driver of risk.
Digging deeper, teams have mapped changes in DNA methylation – one of the best-studied epigenetic marks – across generations in these models. Many altered regions involve genes that regulate metabolism, inflammation, and hormone signaling. Intriguingly, some of the same altered signatures appear in blood from women with PCOS, hinting at shared biology across species.
Inside the Egg: The Epigenetic Toolkit
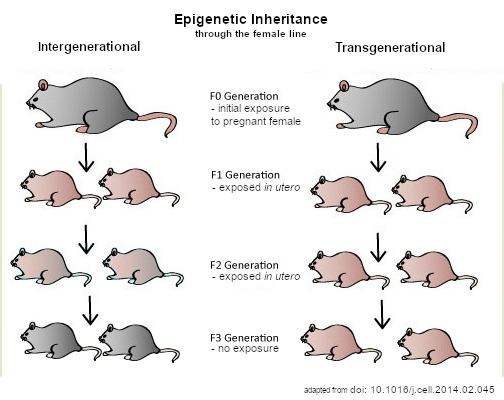
Epigenetic regulation in oocytes is more than methyl marks on DNA; it also includes histone modifications and small RNAs that chaperone early development. During fertilization and the first few days after, most epigenetic marks are wiped clean, but not all – some loci, including imprinted regions and stretches tied to transposable elements, can partially escape the reset. If PCOS disrupts that delicate choreography, the embryo may start life with a skewed playbook. That’s a subtle shift, not destiny, but it can tilt metabolism and hormone responses.
Early embryo studies hint at exactly this kind of mis-tuning. Researchers report pre-activated gene programs and unusual activity in retrotransposon-rich regions in embryos linked to PCOS, a sign that chromatin – the packaging around DNA – opens in the wrong places or at the wrong time. Those early misfires could ripple outward, affecting ovarian function, insulin handling, and even appetite circuits later on.
What the New Study Shows
In 2025, scientists reported a striking twist: in mice with PCOS-like traits, targeted caloric restriction before conception restored oocyte DNA methylation and blocked transmission of the disorder-like features to the next generation. The intervention recalibrated marks on genes tied to insulin secretion and energy sensing, and offspring no longer showed the expected reproductive and metabolic problems. The same study noted parallel methylome disruptions in embryos from women with PCOS and suggested these might be modifiable, raising cautious optimism about translational potential.
This doesn’t mean diet alone “cures” PCOS, nor that people should self-prescribe restrictive plans. It does show that the oocyte’s epigenome is malleable, and that preconception health could carry outsized influence for families facing PCOS risk. Seen this way, the window before pregnancy becomes a powerful lever for change.
Signals Seen in Human Eggs and Embryos
Conference data released this summer added a human-layer perspective: researchers analyzing IVF samples reported disrupted gene regulation and epigenetic marks in egg cells and preimplantation embryos from patients with PCOS. The patterns included changes in key developmental genes and in stretches of DNA that usually keep quiet until later, suggesting timing is off from the start. While preliminary, these observations fit the broader model emerging from animal work.
Importantly, conference findings require peer review and replication, and no clinic should act on them yet. Still, they hint at future IVF workflows that could monitor embryo epigenetic health alongside genetics, and perhaps even test ways to nudge tags back toward typical patterns. That’s a bold vision, but one that’s increasingly plausible as single-cell and low-input sequencing tools mature.
Why It Matters
Most of us learned that inheritance is DNA and everything else is lifestyle, full stop; PCOS is forcing a rethink. The emerging view adds a third dimension: maternal physiology can imprint the egg’s epigenome in ways that echo into the next generation. Compared to traditional risk models that focus on body weight or isolated hormones, this framework explains why some families see PCOS persist even when daughters eat well and exercise. It also helps reconcile why genetics alone, which accounts for only part of PCOS risk, doesn’t tell the whole story.
Clinically, that reframing opens earlier, more precise windows for prevention. If we can identify reversible epigenetic alterations in oocytes, interventions before conception – nutritional, metabolic, or pharmacologic – might blunt risk downstream. For patients, it moves the conversation from blame to biology, which matters for mental health and adherence alike. For health systems, it suggests investing in preconception care could pay back across generations.
Caution and Controversy
Epigenetics can be overhyped, and not every tag is a cause rather than a consequence. Mouse models, while powerful, don’t capture the full complexity of human puberty, diet, or stress, and translating dosage and timing is notoriously tricky. Even when marks correlate with disease, proving causation and safety of interventions takes years. That’s especially true in embryos, where any manipulation must clear high ethical and regulatory bars.
There’s also a vocabulary trap: “intergenerational” effects (seen in direct offspring) aren’t the same as “transgenerational” effects (persisting to grand-offspring with no further exposure). PCOS work includes signals of both, but the field is still sorting definitions and standards. Expect spirited debate over which marks truly escape the embryo’s epigenetic reset and for how long. Skepticism is healthy here; it will make any eventual therapies sturdier.
Global Perspectives

PCOS touches roughly about one in ten women of reproductive age worldwide, but its face varies by region – shaped by diet, pollution, healthcare access, and cultural barriers to seeking help. For families and clinicians in low-resource settings, preconception counseling is often a luxury, not a default. If epigenetic risk truly concentrates before pregnancy, that disparity could widen unless prevention becomes more accessible. The science alone won’t fix that; policy and public health have to keep pace.
On the flip side, epigenetic insights could help tailor interventions to local needs. Nutritional programs, diabetes prevention, and fertility services might integrate preconception checkups that include metabolic and, one day, epigenetic screening. Even modest gains – better glucose control or targeted weight management – could ripple far if they reset oocyte biology at the right moment. It’s a global challenge with a generational payoff.
The Future Landscape
Next-generation tools are racing to meet the questions: single-cell multiomics to profile human oocytes with minimal material, machine learning to flag dangerous methylation patterns, and gentle ways to modulate marks without harming the embryo. Researchers are also exploring pharmacologic routes – from methyl donors to energy-sensing pathway modulators – that might mimic the benefits seen with carefully supervised preconception diet changes. Any of this will require rigorous trials with long follow-up, and transparent registries that track outcomes across childhood.
Ethics will sit front and center, especially if embryo or gamete epigenomes become clinical targets. Noninvasive assays using spent culture media or circulating markers could lower risk, but validation is everything. If the field gets this right, future prenatal care may look less like firefighting and more like careful garden tending – shaping conditions so healthy growth is the default. That’s a quietly radical shift.
How You Can Engage

If PCOS runs in your family, consider preconception checkups that include glucose, lipids, and blood pressure, and talk with your clinician about safe, supported nutrition plans long before you try to conceive. Support participation in longitudinal studies and patient registries; those data help researchers see how epigenetic risk unfolds across lifetimes. If you’re already navigating PCOS, you didn’t cause it – focus on what’s modifiable now, like sleep, stress, and metabolic care, and lean on community for the rest. Advocacy matters too: push for insurance coverage of preconception counseling and equity in fertility care.
From lab bench to kitchen table, the new science suggests your choices can influence tomorrow’s biology without rewriting your genes. That’s empowering, not punitive, and it’s exactly the kind of quiet power families deserve. What small step could you take today that your future self – and perhaps your future child – might thank you for?

Suhail Ahmed is a passionate digital professional and nature enthusiast with over 8 years of experience in content strategy, SEO, web development, and digital operations. Alongside his freelance journey, Suhail actively contributes to nature and wildlife platforms like Discover Wildlife, where he channels his curiosity for the planet into engaging, educational storytelling.
With a strong background in managing digital ecosystems — from ecommerce stores and WordPress websites to social media and automation — Suhail merges technical precision with creative insight. His content reflects a rare balance: SEO-friendly yet deeply human, data-informed yet emotionally resonant.
Driven by a love for discovery and storytelling, Suhail believes in using digital platforms to amplify causes that matter — especially those protecting Earth’s biodiversity and inspiring sustainable living. Whether he’s managing online projects or crafting wildlife content, his goal remains the same: to inform, inspire, and leave a positive digital footprint.