For a long time, mainly for blood cancers such as lymphoma and leukemia CAR T-cell therapy has provided a glimpse of possibility in the treatment of cancer. The latest clinical trial conducted in China has rebutted that notion with a previously unheard-of result against solid tumors. They are a difficult type of cancer to treat. In the quest to treat advanced gastroesophageal and gastric junction cancers, this study has yielded results that could change the face of oncology, by offering an alternative to conventional treatment options that have been unsuccessful. But, even the most effective treatment comes with significant risks, with a number of severe adverse negative effects. This could be the pivotal moment in the fight against tumors that are solid or is there a long and difficult path ahead? This is a thorough overview of the research, the possibilities, and challenges of this revolutionary approach.
The Breakthrough: CAR T-Cells Finally Crack Solid Tumors
In a Phase II clinical trial including 156 advanced gastric or gastroesophageal junction cancers, a CAR T-cell therapy satricabtagene autoleucel (satri-cel) has shown significant success against solid tumors for the first time. Solid tumors lack ideal target antigens and their dense, immunosuppressive microenvironments have caused them to resist CAR T-cell therapy unlike blood cancers. The study was published in The Lancet and presented at ASCO 2025, this study found a 35% success rate for patients who received satri-cel as compared to a mere 4 percent in the group that was treated by conventional treatments, such as the drugs paclitaxel and nivolumab.
The therapy targets CLDN18.2, a protein overexpressed in gastrointestinal tumors. By genetically reprogramming patients’ T-cells to hunt this marker, researchers achieved not just tumor shrinkage but also extended survival by 2.4 months, a significant gain in late-stage cancer. “This is evidence that CAR T-cells can be optimized for solid tumors,” says Lisa Mielke, a cancer researcher at the Olivia Newton-John Cancer Research Institute.
Why Solid Tumors Have Been the “Final Frontier” for CAR T-Cells
CAR T-cell therapy has revolutionized blood cancer treatment, with remission rates exceeding 80% in some leukemias. But solid tumors present unique challenges:
- Hostile Microenvironments: Tumors create physical barriers (like dense connective tissue) and chemical signals that suppress immune cells.
- Antigen Heterogeneity: Unlike blood cancers, which often uniformly express targets like CD19, solid tumors vary widely, allowing cancer cells to evade detection.
- Off-Target Toxicity: Many solid tumor markers are also found in healthy tissues, raising risks of severe side effects.
This trial’s success hinges on CLDN18.2, a tightly restricted antigen mostly found in stomach lining minimizing collateral damage. Still, 99% of patients experienced moderate-to-severe side effects, including cytokine release syndrome (CRS), a dangerous immune overreaction.
The Trial: High Stakes, High Rewards
Patients in the study had exhausted at least two previous treatments. Key conclusions:
- Satri-cel lowered mortality risk by 31% and, for controls, increased median survival to 10.6 months from 8.2 months.
- Some patients observed continuous tumor control, implying CAR T-cells may last longer in solid tumors than first believed.
- Safety Trade-offs: Although side effects were frequent, studies contend they were under control with close observation. “Most were mild,” notes Peking University co-author ChangSong Qi.
Critics warn that still major obstacles are CRS and neurotoxicity. But as Dr. Richard Vile (Mayo Clinic) notes, “Every breakthrough treatment starts with great risk. The secret is to polish it.
The Next Frontier: Earlier Use and Combination Therapies
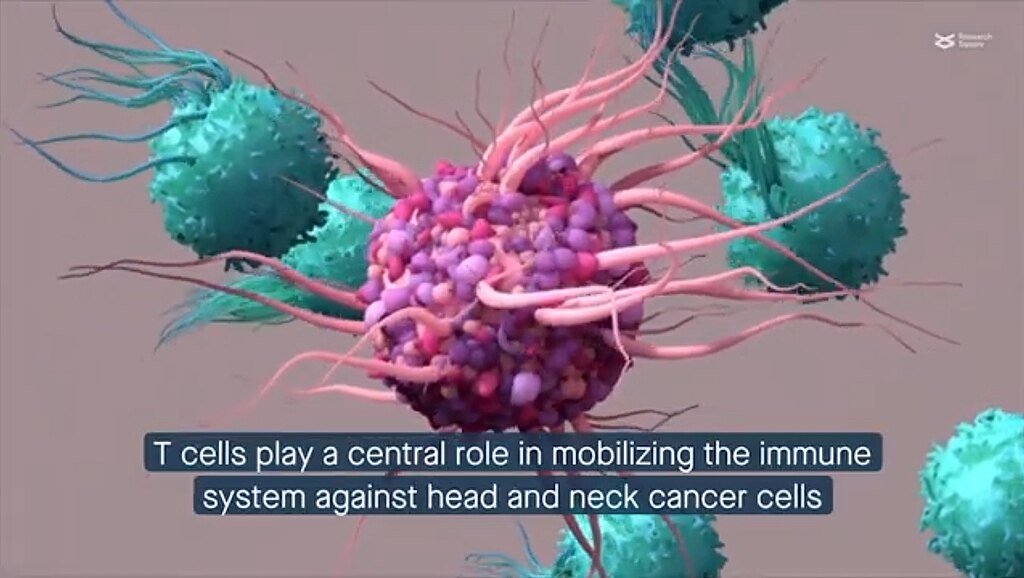
Although CAR T-cells are a last-resort choice right now, researchers think they might travel upstream:
- Qi expects future studies will test satri-cel in less advanced patients, where immune systems are stronger.
- Approaches Combining: Combining CAR T-cells with cancer vaccines or checkpoint inhibitors could improve performance. For example, the mRNA vaccines developed by Moderna and Merck are already demonstrating the ability to increase T-cell responses.
- Versions Off-the- Shelf: Like those under development for myeloma, allogeneic (donor-derived) CAR T-cells could save costs and wait times.
Global Implications: A New Standard of Care?
Once approved, satricel could be the first-in-class treatment available for gastrointestinal cancers, including CLDN18.2-positive ones. However, there are some issues:
- Cost A single dose of CAR T-cell treatments costs $300,000 to $500,000. Accessibility is dependent on the amount of scaling up production.
- The identification of genes that express CLDN18.2 requires an extensive genomic screen, which is currently isn’t being done in a lot of fields at the moment.
- Equity: Similar to other immunotherapies that are available, less-income countries could be affected by delays in the adoption process. Although initiatives such as Cancer Moonshot Cancer Moonshot aim to fill in the gaps, a few variations remain.
The Bigger Picture: What This Means for Cancer Care
Beyond stomach cancer, this study creates opportunities for pan-solid tumor use. Investigators are looking at CAR T-cells for:
- Nearly 95% of early pancreatic tumors, according to a Mayo Clinic study, show clear biomarkers that point to liquid biopsy-guided CAR T approaches.
- Early studies aiming at diffuse midline glioma, a fatal pediatric cancer, show CAR T-cells crossing the blood-brain barrier a historic difficulty.
- New antigen targets for breast and ovarian cancers under study are HER2 and MUC1.
Conclusion: A Cautious Optimism

The satri-cel trial marks a paradigm shift, proving CAR T-cells can combat solid tumors. Yet, as Mielke warns, “This isn’t a cure-all it’s a first step.” With refinements in safety, cost, and accessibility, CAR T-cell therapy could soon join surgery, radiation, and chemotherapy as a pillar of oncology. For now, it offers something equally precious: hope where none existed before.
Sources:

Suhail Ahmed is a passionate digital professional and nature enthusiast with over 8 years of experience in content strategy, SEO, web development, and digital operations. Alongside his freelance journey, Suhail actively contributes to nature and wildlife platforms like Discover Wildlife, where he channels his curiosity for the planet into engaging, educational storytelling.
With a strong background in managing digital ecosystems — from ecommerce stores and WordPress websites to social media and automation — Suhail merges technical precision with creative insight. His content reflects a rare balance: SEO-friendly yet deeply human, data-informed yet emotionally resonant.
Driven by a love for discovery and storytelling, Suhail believes in using digital platforms to amplify causes that matter — especially those protecting Earth’s biodiversity and inspiring sustainable living. Whether he’s managing online projects or crafting wildlife content, his goal remains the same: to inform, inspire, and leave a positive digital footprint.




