Across biology, a handful of species keep rewriting what we think we know about cancer – resisting it, reshaping it, or enduring in spite of it. Their stories aren’t fairy tales of invincibility; they’re tougher and far more useful. Each animal here reveals a different strategy cells can use to survive stress, repair damage, or shut down dangerous growth. For researchers racing to outsmart tumors, these natural experiments offer blueprints hiding in plain sight. The puzzle is turning wild resilience into humane, effective therapies that help people now, not just someday.
Naked Mole-Rat: The Hidden Clues Beneath the Underground

It sounds like a rumor from a desert burrow: a small, wrinkled rodent that almost never develops cancer. The naked mole-rat’s secret sits in its extracellular matrix, where unusually large chains of hyaluronan create a dense, slippery environment that pushes cells to stop dividing when they get too close. That reinforced “personal space” links to a belt-and-suspenders form of contact inhibition, backed by cell-cycle brakes that engage earlier than in typical rodents. The species still isn’t immune – rare tumors do occur – but the bar for malignant growth is set much higher.
In practical terms, that matrix acts like thick winter rubber on a racetrack, forcing speed limits that most cells respect. Scientists have begun mimicking these mechanics in engineered tissues to see whether tightening the cellular neighborhood can discourage precancerous growth. The idea is simple: if you can alter the physical rules of the track, you can slow the race before it turns dangerous.
African Elephant: Peto’s Paradox in Motion

Elephants carry vastly more cells than we do, yet they don’t have sky-high cancer rates; that’s Peto’s paradox in the wild. Their genomes hold extra copies of the tumor suppressor gene TP53 and other damage-response tricks, making elephant cells quick to trigger self-destruction when DNA errors pile up. Think of it as a building with dozens of smoke detectors instead of a few – false alarms happen, but catastrophic fires are rarer. That bias toward rapid apoptosis trades a bit of growth efficiency for safety.
Researchers are now probing how those extra gene copies cooperate with elephant-specific regulatory switches. The aim isn’t to copy a pachyderm genome into people, but to learn how to harden human cellular fire codes so dangerous cells exit early, quietly, and for good.
Bowhead Whale: Longevity Without the Expected Cancer Burden

Bowhead whales can live for two centuries, a span that should give cancer plenty of chances to emerge. Instead, genomic studies point to strengthened DNA repair, controlled cell cycling, and metabolic traits that reduce chronic cellular stress. The whales’ slow pace of life – low body temperature, glacial growth – also keeps damage rates down, like dimming the lights to extend the bulb’s life. It’s not one magic mutation; it’s an orchestra of small defenses playing in tune over decades.
For cancer prevention, that matters: long-term risk is a compounding problem, and bowheads show how lowering baseline stress shifts the curve. Translating that idea into humans means targeting chronic inflammation, improving repair fidelity, and designing therapies that prioritize long-run cellular housekeeping, not just short-run tumor kill.
Tasmanian Devil: Evolution Fights Back Against a Transmissible Cancer

Tasmanian devils faced a nightmare – a contagious facial tumor that spreads through biting and at one point devastated populations. Yet in just a few devil generations, natural selection began favoring animals with immune and genetic traits that blunt disease progression. Field data show local rebounds, and experimental vaccines are being tested alongside conservation breeding and targeted releases. This is resilience at the population level, where ecology, behavior, and genetics align to push back.
The devil’s story reframes cancer not only as a cell-autonomous crisis but as a social disease with ecological levers. For human oncology, it strengthens the case for prevention strategies that combine immunology with behavior change – limit the opportunities for “transmission-like” risks such as carcinogenic exposures, and amplify the immune cues that flag dangerous cells sooner.
Tardigrade: DNA Shielding in an Unlikely Survivor
Tardigrades shrug off radiation and vacuum-level stress by protecting DNA from shattering, aided by proteins like Dsup that bind chromatin and reduce damage. In human cells, engineered expression of Dsup can dampen radiation-induced breaks, offering a provocative proof of concept. That doesn’t mean tardigrade biology is ready for the clinic, but it does point to an approach: reinforce the genome during therapy to spare healthy tissue while you target the tumor. It’s like handing cells a shock absorber before the road turns rocky.
Oncologists are watching because radiotherapy and some chemotherapies hinge on DNA damage. If we can shield normal cells selectively – time-limited, tightly controlled – we may push doses higher where needed or reduce side effects where possible, both of which change the risk–benefit equation.
Planarian Flatworm: Regeneration with Built-In Safeguards

Slice a planarian and it rebuilds itself, powered by stem-like neoblasts that can become any tissue. Unlimited growth is dangerous territory, yet planarians keep it under control with pathways that police proliferation and trigger cell death when patterns misfire. Researchers use this system to dissect how regeneration avoids tipping into tumorigenesis, mapping regulators that balance repair with restraint. It’s regeneration with a seatbelt, not a joyride without brakes.
The lessons land squarely in oncology and wound healing. If we can borrow the rules that keep planarian growth coordinated – timing cues, positional information, damage checkpoints – we might support organ repair after surgery while reducing the chance of tumor relapse at the same site.
Hydra: Near-Ageless Bodies and Tumor Lessons from Simplicity
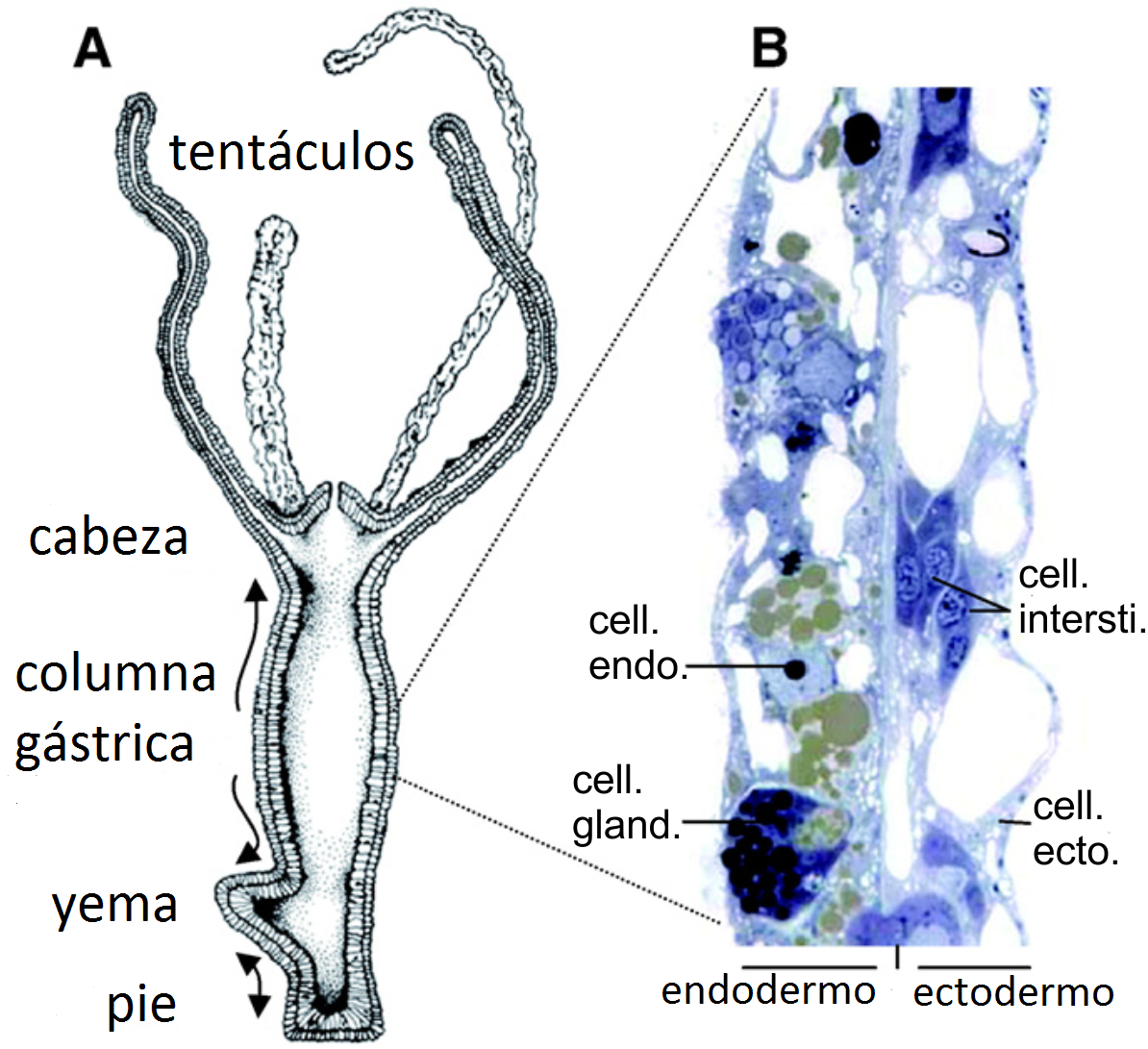
Hydra don’t seem to age in the usual way, thanks to constant stem cell renewal that maintains tissues indefinitely. That fountain-of-youth status makes deviations stand out: when hydra develop tumor-like overgrowths, the cellular missteps are easier to track in a simple body plan. Studies point to community rules – how cell types talk, how microbes influence signaling – that either stabilize the system or let it run away. In other words, resilience here is social, not just individual.
For cancer, that suggests interventions targeting the microenvironment and the microbiome, not only the malignant cells themselves. Recreating hydra’s stable neighborhood – clear roles, steady signals – could help us design therapies that restore order rather than fight endless battles with every rogue cell.
Why It Matters
Traditional cancer models, especially short-lived lab rodents, give speed and control but compress the timeline of disease. The animals above add what those models can’t: stress-tested solutions that have survived evolutionary peer review. Their strategies cluster into themes – enhanced damage sensing, strict growth brakes, fortified extracellular matrices, and environmentally tuned metabolism – that together lower the odds of malignant runaway. Comparative biology turns these themes into testable hypotheses, closing the gap between what works in a dish and what survives in a body.
Consider the practical signals: tougher contact inhibition from naked mole-rats, more vigilant DNA checkpoints from elephants, long-horizon maintenance from bowheads, and immune recalibration from devils. Each idea maps to a clinical lever – prevention that hardens tissues against initiation, diagnostics that flag dangerous clones earlier, and therapies that push malignant cells toward orderly exit instead of chaotic survival.
The Future Landscape
Next-gen sequencing is now cheap enough to widen the animal roster, pairing genomes with single-cell atlases of development, immunity, and aging. Organoids grown from human tissues can be “tuned” with lessons from wildlife – stiffer matrices, altered growth cues, engineered damage responses – to trial resilience strategies before they reach patients. Gene editors and programmable RNA tools may emulate elephant-like alarms transiently, timed to protect healthy cells during treatment. At the same time, AI models that learn across species could spot conserved weak points tumors can’t easily reroute.
The hurdles are clear: safety, specificity, and equity. We’ll need delivery systems that act locally and briefly, regulatory paths that keep pace with platform therapies, and study designs that include diverse patients so benefits don’t skip communities already underserved by cancer care.
Conclusion

Support the research pipeline that connects field biology to the clinic – comparative oncology centers, data-sharing initiatives, and registries that track outcomes over years, not months. Back conservation efforts for species that teach us most; protecting elephants or devils is also protecting a library of anti-cancer ideas. Stay skeptical of miracle cures and seek evidence-backed trials when considering cutting-edge therapies, especially those borrowing from unusual biology. If you’re in a position to participate, enroll in longitudinal studies that focus on prevention and early detection, where resilience lessons are poised to have the biggest payoff.
Finally, share what you learn: debunk myths, highlight real advances, and point friends toward reputable cancer centers and patient networks. Small actions add up, especially when they keep attention – and funding – focused on science that endures.

Suhail Ahmed is a passionate digital professional and nature enthusiast with over 8 years of experience in content strategy, SEO, web development, and digital operations. Alongside his freelance journey, Suhail actively contributes to nature and wildlife platforms like Discover Wildlife, where he channels his curiosity for the planet into engaging, educational storytelling.
With a strong background in managing digital ecosystems — from ecommerce stores and WordPress websites to social media and automation — Suhail merges technical precision with creative insight. His content reflects a rare balance: SEO-friendly yet deeply human, data-informed yet emotionally resonant.
Driven by a love for discovery and storytelling, Suhail believes in using digital platforms to amplify causes that matter — especially those protecting Earth’s biodiversity and inspiring sustainable living. Whether he’s managing online projects or crafting wildlife content, his goal remains the same: to inform, inspire, and leave a positive digital footprint.